Published online Jan 21, 2005. doi: 10.3748/wjg.v11.i3.344
Revised: April 8, 2004
Accepted: May 13, 2004
Published online: January 21, 2005
AIM: To investigate the expression pattern of epithelial cell adhesion molecule (Ep-CAM) on normal and malignant colon tissues to evaluate its diagnostic and therapeutic significance.
METHODS: cDNA encoding Ep-CAM extracellular domain was cloned by reverse transcription-polymerase chain reaction (RT-PCR) from excised malignant colon tissues and inserted into a glutathione S-transferase (GST)-tagged vector. Ep-CAM-GST fusion protein was induced by isopropyl-β-D-thiogalactopyranoside (IPTG) and purified with glutathione-sepharose. The Ep-CAM-GST fusion protein was mixed with Freund’s adjuvant and Balb/c mice were immunized with it. Sp2/0 myeloma cells were fused with the spleen cells of the immunized mice. After having selected by indirect ELISA, the anti-Ep-CAM monoclonal antibodies (MAbs) were generated and the corresponding ascites were obtained. Finally, the human colon carcinoma tissue array prepared from seventy individual patients was stained with the anti-Ep-CAM MAbs.
RESULTS: The isolated Ep-CAM cDNA sequence was identical to the data in GenBank. The expressed fusion protein was almost soluble and had a molecular weight (MW) of 53 ku. Four MAbs against Ep-CAM were obtained and designated as FMU-Ep1, FMU-Ep2, FMU-Ep3 and FMU-Ep4 respectively. Among them, FMU-Ep4 could recognize the natural Ep-CAM on Colo205 and SW480 cells, and all of them could be used for immunohistochemical staining of tissue sections. It was found that Ep-CAM was distributed differently in normal and various malignant colon tissues, including squamous cell carcinoma, signet-ring cell carcinoma and adenocarcinoma. In normal colon gland epithelia, Ep-CAM antigen was mainly distributed on the basolateral membrane and in the region between the basolateral membrane and the cytoplastic part near the nuclei, whereas the expression pattern of colon malignancies was mainly on the whole surface of epithelia and the expression was much higher than the normal colon tissues. The staining pattern of tissue array showed in adenocarcinoma and papillary adenocarcinoma, and the expression of Ep-CAM was increased from grade I to grade III.
CONCLUSION: MAbs against Ep-CAM might be useful for research on the structure and function of Ep-CAM and may have diagnostic and therapeutic value to various colon carcinomas.
- Citation: Xie X, Wang CY, Cao YX, Wang W, Zhuang R, Chen LH, Dang NN, Fang L, Jin BQ. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J Gastroenterol 2005; 11(3): 344-347
- URL: https://www.wjgnet.com/1007-9327/full/v11/i3/344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i3.344
Epithelial cell adhesion molecule (Ep-CAM), a 40 ku transmembrane glycoprotein with two epithelial growth factor (EGF)-like domains, is broadly expressed on normal epithelial cells mediating the Ca2+-independent homotypic cell-cell adhesions as well as on some epithelia-derived neoplasms[1]. It has been demonstrated that the expression level of Ep-CAM is closely related to the differentiation of various tumors such as bladder carcinoma, breast carcinoma, cervical squamous carcinoma and oral mucosal dysplasia[2-5]. Ep-CAM is encoded by GA733-2 gene located on human chromosome 4q[6]. Low affinity monoclonal antibody (MAb) against Ep-CAM 17-1A has been successfully used in Germany for breast and colon carcinoma therapy[7,8].
Freshly excised colon cancer tissue was washed with 0.15 mol/L PBS and resuspended in 200 μL of lysis buffer (5 mL/L Triton X-100, 10 mol/L Tris-HCl, pH 8, 0.1 mmol/L EDTA, 0.32 mol/L sucrose, 3 mmol/L MgCl2, 1 mmol/L PMSF (Sigma Chemical Co., St. Louis, MO), and 10 mg/L leupeptin (Sigma Chemical Co., St. Louis, MO). The tissue was ground using a mortar and pestle, and solubilized in RNA extraction solution (TRIzol, Invitrogen, San Diego, CA). Total RNA was isolated using chloroform, and precipitated with isopropanol. The resulting 1 μg of RNA was used as template for single-strand cDNA synthesis with 20 U avian myeloblastosis virus (AMV) reverse transcriptase (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. Primers for PCR amplification were synthesized by Shanghai Sangon Biological Engineering and Technology Service Co. Ltd. Primers were as follows: sense, 5’-CAGGAAGAATGTGTCTGTG-3’ and antisense, 5’-CCACGCACACACATTTGTAA-3’ for Ep-CAM; sense, 5’-GGCACCACACCTTCTACAATG-3’ and antisense, 5’-CAGGAAGGAAGGTTGGAAGAG-3’ for β-actin. PCR reaction mixture was subjected to pre-denaturation at 94 °C for 5 min followed by 31 amplification cycles each consisting of denaturation at 94 °C for 1 min, annealing at 58 °C for 1 min and extension at 72 °C for 3 min, and then a final elongation cycle at 72 °C for 10 min was performed with thermo-DNA polymerase Pfu (Stratagene, La Jolla, CA). Products of PCR were electrophoresed on 10 g/L agarose gels and cloned into the pGEM-3Zf, which was digested with SmaI. cDNA clones were sequenced using automated DNA sequencing facilities from Shanghai Sangon Biological Engineering and Technology Service Co. Ltd.
Murine myeloma cell line Sp2/0, human colon carcinoma cell lines Colo205 and SW480 were incubated in RPMI 1640 (Sigma Chemical Co., St. Louis, MO) containing 100 mL/L fetal calf serum (FCS, GIBCO-Invitrogen Co., San Diego, CA). Hybridomas were incubated in RPMI 1640 supplemented with 20 mL/L FCS.
The cDNA clone encoding the extracellular domain of Ep-CAM was subcloned with pyrobest polymerase (Takara, China) and inserted into a pGEX-4T-3 vector (Amersham Pharmacia Biotech, Piscataway, NJ). The corresponding protein was expressed in Escherichia coli as glutathione S-transferase (GST) fusion protein induced by isopropyl-β-D-thiogalactopyranoside (IPTG, Sigma Chemical Co., St. Louis, MO) and purified with glutathione-sepharose.
Female Balb/c mice (4 wk old) were immunized with 40 μg of purified Ep-CAM-GST fusion protein in complete Freund’s adjuvant (Sigma Chemical Co., St. Louis, MO) by subcutaneous (sc) injection. Subsequently, immunizations were carried out twice with 40 μg of antigen in incomplete Freund’s adjuvant (Sigma Chemical Co., St. Louis, MO) by sc and intraperitoneal (ip) injection respectively, at 3 wk intervals. Ten days after the third immunization, mice were bled from caudal vein and the serum titer was determined by ELISA. The immunized mice were boosted with 20 μg of Ep-CAM-GST fusion protein by ip injection three days before fusion. Splenocytes from immunized mice and Sp2/0 myeloma cells were fused according to the general procedure in the presence of polyethylene glycol (PEG, MW 4000, Merck, Germany)[9]. Positive hybrids were selected by indirect ELISA and subcloned 3 times using the limiting dilution method. MAbs were produced either from supernatants of the hybridoma culture or from ascites fluid of Balb/c mice intraperitoneally injected with hybridoma. Immunoglobulin (Ig) isotypes were identified by using an isotyping kit (Pierce, Rockford, IL).
ELISA was performed on 96-well plates (NUNC, Nagel Inc., Roskilde, Denmark), which were coated with 5 mg/L Ep-CAM-GST or GST respectively, in coating buffer (0.05 mol/L carbonate/bicarbonate buffer, pH 9.6) and incubated overnight at 4 °C. After washing three times with PBS/0.5 mL/L Tween-20, the supernatants of hybridoma culture or various dilutions of corresponding murine ascites were added and the plates were incubated for 1 h at 37 °C. The plates were washed three times and the working dilution (1:2000) of horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (DAKO, Glostrup, Denmark) was added. After incubated at 37 °C for 1 h and three more washes, the substrate 2,2’-azino-bis (3-ethylbenzo thiasoline-6-sulfonic acid) (ABTS, Sigma Chemical Co., St. Louis, MO) was added to each well and incubated for 15 min, and then the absorbance at 410 nm (A410 nm) was detected on a micro-plate reader.
The reported Ep-CAM positive cell line Colo205 was incubated with anti-Ep-CAM MAbs or negative control anti-staphylococcal enterotoxin B (SEB) mAb (IgG1) at 4 °C for 30 min, respectively. After washing twice, the cells were suspended in Dulbecco phosphate-buffered saline (DPBS) containing a working dilution of fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG antibodies (Sigma) and incubated for 30 min at 4 °C. Cells were washed, fixed and then analyzed on a FACScan cytometer (ELITE ESP, Coulter Inc., Miami, FL).
Formalin-fixed and paraffin-embedded normal or malignant human colon tissue sections were obtained from the tissue bank of the Department of Pathology at the XJ Hospital (Xi’an, Shaanxi, China). All tissues were sampled from surgical specimens within 2 h of resection. Three-micrometer thick sections were mounted on poly-L-lysine-coated slides, followed by deparaffinization in xylene and dehydration in graded alcohol. The endogenous peroxidase activity was blocked by an incubation with 3 mL/L H2O2 in methanol for 30 min. Tissue sections were subjected to antigen retrieval by boiling in 0.01 mol/L sodium-citrate (pH 6) for 10 min in a microwave oven. After blocked with 15 mL/L normal goat serum for 1 h, immunohistochemistry assay was carried out with an Elivision plus staining kit (Maixub-bio, Fuzhou, China) according to its manual. In brief, tissue sections were stained with MAb against Ep-CAM with concentration of 50 μg/L for 1 h at room temperature and washed three times, after which an enhancer was added for 30 min, followed by three washes before HRP-conjugated goat anti-mouse was added and washed another three times. Finally, diaminobenzidine (DAB) was added for colouring. Human colon carcinoma tissue array prepared from seventy individual patients supplied by Cybrdi Corporation (Xi’an, China) was stained with the same procedure mentioned above.
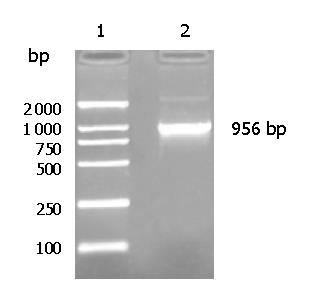
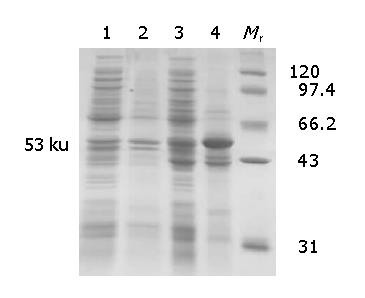
Ep-CAM cDNA was successfully cloned from the malignant colon tissue (Figure 1) and its sequencing result was identical to the data in GenBank. The optimal condition inducing the soluble Ep-CAM-GST fusion protein to the greatest extent was at 37 °C for 7 h with 100 nmol/L IPTG. The SDS-PAGE results showed the Ep-CAM-GST fusion protein had a molecular weight (MW) of 53 ku (Figure 2).
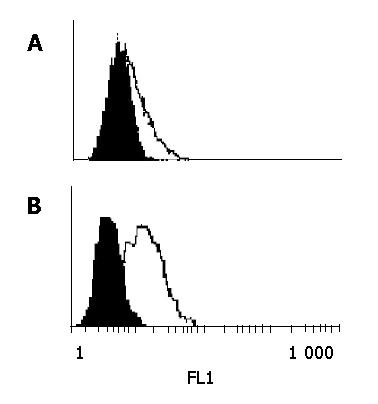
Four hybridoma clones secreting MAbs to Ep-CAM were obtained by the routine hybridoma technology and designated as FMU-Ep1, FMU-Ep2, FMU-Ep3 and FMU-Ep4 respectively. The clone name, isotype, titers, ability of immunohistochemical staining, and reaction with membrane Ep-CAM in FCM are listed in Table 1. Among them, FMU-Ep4 could recognize the natural Ep-CAM on Colo205 and SW480 cells (Figure 3), and all of them could be used for immunohistochemical staining on tissue sections.
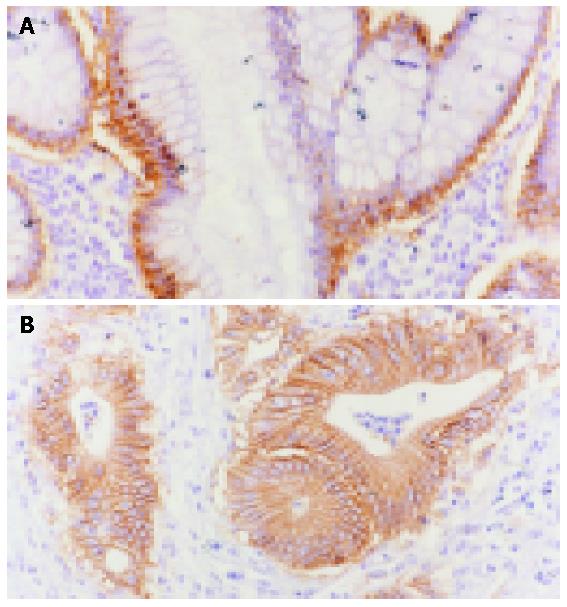
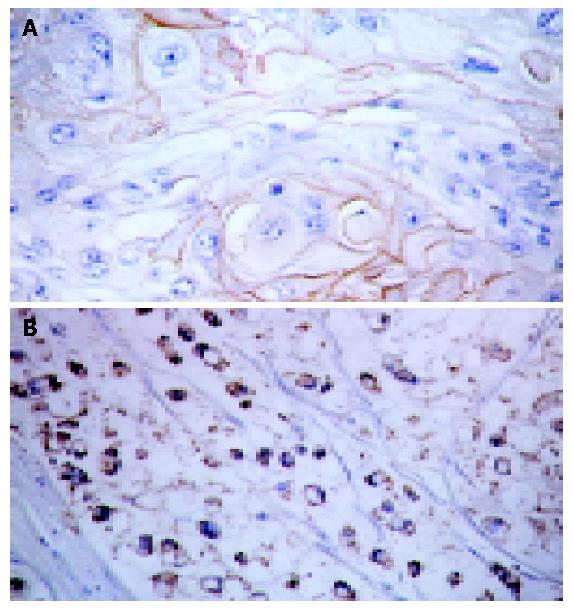
Ep-CAM mAb FMU-Ep1 was selected for the immunohistochemistry staining. In the normal colon tissues, Ep-CAM antigen mainly distributed on the basolateral membrane and the region between the basolateral membrane of colon gland epithelia and the cytoplastic part near the nucli, but not all the junctions of cells, whereas the expression pattern of colon malignancies was mainly on the whole surface of epithelia and the expression was much higher than the normal colon tissues (Figure 4).
Seventy-two tissue dots were collected from 70 individual patients with colon carcinoma tissue array, including adenocar-cinoma (grades I to III), papillary adenocarcinoma (grades I to III), signet-ring and squamous cell carcinomas as well as other non-tumor tissues such as fibrous, fatty and mucous membrane inflammation tissues, smooth muscle and B cell lymphoma. We found that Ep-CAM was expressed only on the epithelia-origined normal and malignant tissues. Among them, Ep-CAM was positive on cell surface and cytoplastic region of signet-ring carcinoma cells and only on the surface of squamous cells (Figure 5). In adenocarcinoma and papillary adenocarcinoma, the expression of Ep-CAM was increased from grades I to III.
Epithelial cell adhesion molecule (Ep-CAM) has been taken as a target of biological therapy[7,8,10] and a tool in surgical pathology[11]. Studies have shown that Ep-CAM is mainly distributed on human basolateral cell membranes of all simple, pseudo-stratified, transitional epithelia and some specific epithelia-like hepatocytes, but not in cells of lymphoid origin[12,13]. In the gastrointestinal tract, gastric epithelium expresses very low levels of Ep-CAM, higher levels in small intestine and the highest level in colon[14]. A previous research had revealed that Ep-CAM mediates Ca2+-independent homotypic cell-cell adhesions[15]. Formation of Ep-CAM-mediated adhesions has a negative regulatory effect on adhesions mediated by classic cadherins, which may have strong effects on the differentiation and growth of epithelial cells[16,17].
In gastrointestinal tract carcinoma, malignant proliferation of cancer cells are frequently associated with Ep-CAM expression in gastrointestinal tract carcinomas, such as small intestine carcinoma and colorectal adenocarcinoma[18,19]. Our research has revealed that the expression of Ep-CAM in human colon adenocarcinoma is similar to that in mice and its expression level is negatively related to carcinoma differentiation. On tissue array, the expression of Ep-CAM on adenocarcinoma and papillary adenocarcinoma is increased from grade I to grade III. Unlike the normal colon tissues, both basolateral and apical membranes are Ep-CAM positive.
In our experiments, Ep-CAM was expressed as a prokaryotic fusion protein form, which was then taken as an immunogen to prepare monoclonal antibodies. The four strains of MAbs could recognize Ep-CAM denatured by formalin, and could be selected as potential pathological tools for their immunohistochemical staining sensitivity. Only one strain could recognize natural Ep-CAM. It might be due to two possible reasons. One is that Ep-CAM is a highly glycosylated molecule[20], and the prokaryotic and eukaryotic fusion protein differ in structure. The other is that the extent of glycosylation might be different in malignant and normal colon epithelial cells, which causes the final distinct structure.
After 7 years of following up patients with Duke’C colorectal carcinoma who underwent curative surgery and adjuvant therapy with edrecolomab, a 32% reduction in mortality and a 23% reduction in recurrence were found[7]. Adjuvant treatment with low-affinity Ep-CAM MAb 17-1A successfully increased the 5-year survival rate after surgical treatment of breast and colon carcinomas in Germany for decades[21]. In recent years, the incidence of colorectal cancer has been increasing rapidly in China, making it the fourth leading cause of cancer mortality in China[22]. Preparation of a set of MAbs against Ep-CAM may contribute to several potential future usages, including study on the structure and function of Ep-CAM as well as the diagnosis and therapy of some epithelial-derived cancers.
Edited by Kumar M and Wang XL
| 1. | Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl). 1999;77:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 410] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Zorzos J, Zizi A, Bakiras A, Pectasidis D, Skarlos DV, Zorzos H, Elemenoglou J, Likourinas M. Expression of a cell surface antigen recognized by the monoclonal antibody AUA1 in bladder carcinoma: an immunohistochemical study. Eur Urol. 1995;28:251-254. [PubMed] |
| 3. | Tsubura A, Senzaki H, Sasaki M, Hilgers J, Morii S. Immunohistochemical demonstration of breast-derived and/or carcinoma-associated glycoproteins in normal skin appendages and their tumors. J Cutan Pathol. 1992;19:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Litvinov SV, van Driel W, van Rhijn CM, Bakker HA, van Krieken H, Fleuren GJ, Warnaar SO. Expression of Ep-CAM in cervical squamous epithelia correlates with an increased proliferation and the disappearance of markers for terminal differentiation. Am J Pathol. 1996;148:865-875. [PubMed] |
| 5. | High AS, Robinson PA, Klein CE. Increased expression of a 38kd cell-surface glycoprotein MH99 (KS 1/4) in oral mucosal dysplasias. J Oral Pathol Med. 1996;25:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Linnenbach AJ, Seng BA, Wu S, Robbins S, Scollon M, Pyrc JJ, Druck T, Huebner K. Retroposition in a family of carcinoma-associated antigen genes. Mol Cell Biol. 1993;13:1507-1515. [PubMed] |
| 7. | Riethmüller G, Holz E, Schlimok G, Schmiegel W, Raab R, Höffken K, Gruber R, Funke I, Pichlmaier H, Hirche H. Monoclonal antibody therapy for resected Dukes' C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788-1794. [PubMed] |
| 8. | Kirchner EM, Gerhards R, Voigtmann R. Sequential immunochemotherapy and edrecolomab in the adjuvant therapy of breast cancer: reduction of 17-1A-positive disseminated tumour cells. Ann Oncol. 2002;13:1044-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liu F, Liu Y, Jin B, Dong BQ, Zhu Y. Preparation and characterization of monoclonal antibodies against GAM protein: a novel gp130-associated molecule. Hybridoma. 1999;18:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Haller DG. Update of clinical trials with edrecolomab: a monoclonal antibody therapy for colorectal cancer. Semin Oncol. 2001;28:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Winter MJ, Nagtegaal ID, van Krieken JH, Litvinov SV. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol. 2003;163:2139-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Momburg F, Moldenhauer G, Hämmerling GJ, Möller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987;47:2883-2891. [PubMed] |
| 13. | de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Schiechl H, Dohr G, Eherer A. Immunohistochemical localization and characterization of a protein from the basolateral membrane of rat small intestine epithelium using monoclonal antibody GZ-1. J Histochem Cytochem. 1986;34:1659-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 388] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Winter MJ, Nagelkerken B, Mertens AE, Rees-Bakker HA, Briaire-de Bruijn IH, Litvinov SV. Expression of Ep-CAM shifts the state of cadherin-mediated adhesions from strong to weak. Exp Cell Res. 2003;285:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Balzar M, Bakker HA, Briaire-de-Bruijn IH, Fleuren GJ, Warnaar SO, Litvinov SV. Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule. Mol Cell Biol. 1998;18:4833-4843. [PubMed] |
| 18. | Zaloudik J, Basak S, Nesbit M, Speicher DW, Wunner WH, Miller E, Ernst-Grotkowski C, Kennedy R, Bergsagel LP, Koido T. Expression of an antigen homologous to the human CO17-1A/GA733 colon cancer antigen in animal tissues. Br J Cancer. 1997;76:909-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Daly T, Royal RE, Kershaw MH, Treisman J, Wang G, Li W, Herlyn D, Eshhar Z, Hwu P. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 2000;7:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Chong JM, Speicher DW. Determination of disulfide bond assignments and N-glycosylation sites of the human gastrointestinal carcinoma antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J Biol Chem. 2001;276:5804-5813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Armstrong A, Eck SL. EpCAM: A new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Qing SH, Rao KY, Jiang HY, Wexner SD. Racial differences in the anatomical distribution of colorectal cancer: a study of differences between American and Chinese patients. World J Gastroenterol. 2003;9:721-725. [PubMed] |