Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4560
Revised: September 1, 2004
Accepted: September 4, 2004
Published online: August 7, 2005
AIM: To study the portal hemodynamics and their relationship with the size of esophageal varices seen at endoscopy and to evaluate whether these Doppler ultrasound parameters might predict variceal bleeding in patients with liver cirrhosis and portal hypertension.
METHODS: One hundred and twenty cirrhotic patients with esophageal varices but without any previous bleeding were enrolled in the prospective study. During a 2-year observation period, 52 patients who had at least one episode of acute esophageal variceal hemorrhage constituted the bleeding group, and the remaining 68 patients without any previous hemorrhage constituted the non-bleeding group. All patients underwent endoscopy before or after color Doppler-ultrasonic examination, and images were interpreted independently by two endoscopists. The control group consisted of 30 healthy subjects, matched to the patient group in age and gender. Measurements of diameter, flow direction and flow velocity in the left gastric vein (LGV) and the portal vein (PV) were done in all patients and controls using color Doppler unit. After baseline measurements, 30 min after oral administration of 75 g glucose in 225 mL, changes of the diameter, flow velocity and direction in the PV and LGV were examined in 60 patients with esophageal varices and 15 healthy controls.
RESULTS: The PV and LGV were detected successfully in 115 (96%) and 105 (88%) of 120 cirrhotic patients, respectively, and in 27 (90%) and 21 (70%) of 30 healthy controls, respectively. Among the 120 cirrhotic patients, 37 had F1, 59 had F2, and 24 had F3 grade varices. Compared with the healthy controls, cirrhotic group had a significantly lower velocity in the PV, a significantly greater diameter of the PV and LGV, and a higher velocity in the LGV. In the cirrhotic group, no difference in portal flow velocity and diameter were observed between patients with or without esophageal variceal bleeding (EVB). However, the diameter and blood flow velocity of the LGV were significantly higher for EVB (+) group compared with EVB (-) group (P<0.01). Diameter of the LGV increased with enlarged size of varices. There were differences between F1 and F2, F1 and F3 varices, but no differences between F2 and F3 varices (P = 0.125). However, variceal bleeding was more frequent in patients with a diameter of LGV >6 mm. The flow velocity in the LGV of healthy controls was 8.70±1.91 cm/s (n = 21). In patients with liver cirrhosis, it was 10.3±2.1 cm/s (n = 12) when the flow was hepatopetal and 13.5±2.3 cm/s (n = 87) when it was hepatofugal. As the size of varices enlarged, hepatofugal flow velocity increased (P<0.01) and was significantly different between patients with F1 and F2 varices and between patients with F2 and F3 varices. Variceal bleeding was more frequent in patients with a hepatofugal flow velocity >15 cm/s (32 of 52 patients, 61.5%). Within the bleeding group, the mean LGV blood flow velocity was 16.6±2.62 cm/s. No correlation was observed between the portal blood flow velocity and EVB. In all healthy controls, the flow direction in the LGV was hepatopetal, toward the PV. In patients with F1 varices, flow direction was hepatopetal in 10 patients, to-and-fro state in 3 patients, and hepatofugal in the remaining 18. The flow was hepatofugal in 91% patients with F2 and all F3 varices. Changes in diameter of the PV and LGV were not significant before and after ingestion of glucose (PV: 1.41±1.5 cm before and 1.46±1.6 cm after; LGV: 0.57±1.7 cm before and 0.60±1.5 cm after). Flow direction in the LGV was hepatopetal and to-and-fro in 16 patients and hepatofugal in 44 patients before ingestion of glucose. Flow direction changed to hepatofugal in 9 of 16 patients with hepatopetal and to-and-fro blood flow after ingestion of glucose. In 44 patients with hepatofugal blood flow in the LGV, a significant increase in hepatofugal flow velocity was observed in 38 of 44 patients (86%) with esophageal varices. There was a relationship between the percentage changes in flow velocity and the size of varices. Patients who responded excessively to food ingestion might have a high risk for bleeding. The changes of blood flow velocity in the LGV were greater than those in the PV (LGV: 28.3±26.1%, PV: 7.2±13.2%, P<0.01), whereas no significant changes in the LGV occurred before and after ingestion of glucose in the control subjects.
CONCLUSION: Hemodynamics of the PV is unrelated to the degree of endoscopic abnormalities in patients with liver cirrhosis. The most important combinations are endoscopic findings followed by the LGV hemodynamics. Duplex-Doppler ultrasonography has no value in the identification of patients with cirrhosis at risk of variceal bleeding. Hemodynamics of the LGV appears to be superior to those of the PV in predicting bleeding.
- Citation: Li FH, Hao J, Xia JG, Li HL, Fang H. Hemodynamic analysis of esophageal varices in patients with liver cirrhosis using color Doppler ultrasound. World J Gastroenterol 2005; 11(29): 4560-4565
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4560
Esophageal variceal bleeding (EVB) is a potentially deadly complication in patients with liver cirrhosis and portal hypertension[1-4]. In patients with cirrhosis, the incidence of esophageal varices ranges from 35% to 80% and approximately a third of patients with esophageal varices experience variceal bleeding, and up to 70% of the survivors have one or more additional episodes of bleeding[5]. The ultrasonographic examination is a simple, inexpensive, accurate, and noninvasive technique. It has been widely used to investigate the relationship between EVB and hemodynamics associated with portal hypertension and liver cirrhosis[6-9]. However, no consistent results have been reported yet. In this study, we investigated the hemodynamic features of the portal vein (PV) and left gastric vein (LGV) before and after oral glucose (75 mg), and evaluated whether these Doppler ultrasound parameters might predict variceal bleeding in patients with liver cirrhosis.
One hundred and twenty cirrhotic patients with esophageal varices without any previous bleeding (mean age 57.6±6.8 years; 42 females and 78 males) were enrolled in the prospective study. During a 2-year observation period, 52 patients who had at least one episode of acute esophageal variceal hemo-rrhage constituted the bleeding group, and the remaining 68 patients without any previous hemorrhage constituted the non-bleeding group. The diagnosis of cirrhosis was based on clinical and imaging findings or pathologic findings.
The control group consisted of 30 healthy subjects, matched to the patient group in age (mean age 53.8±6.7 years) and gender (10 females and 20 males). None of the controls had a history or clinical evidence of liver disease. All subjects provided written informed consent prior to participation.
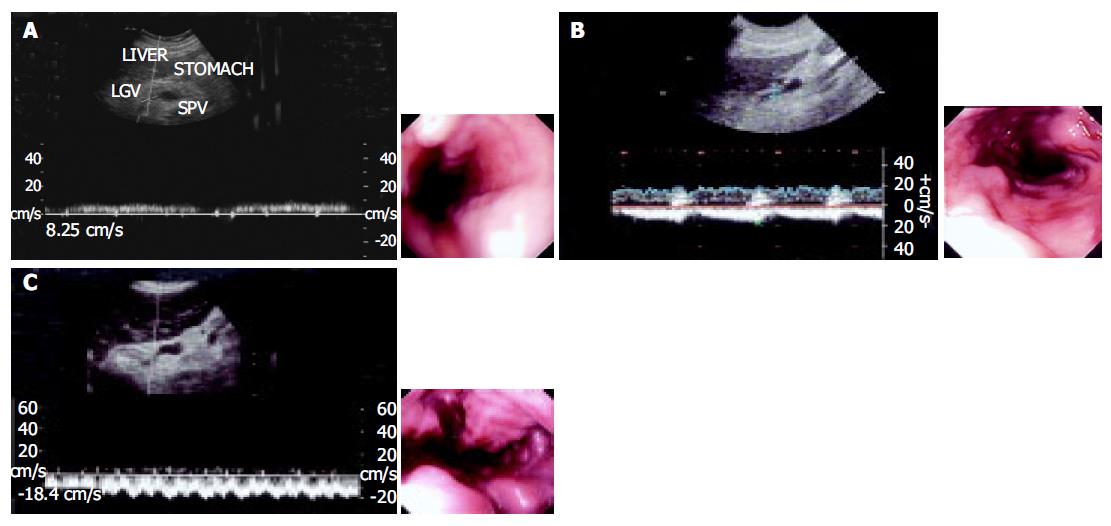
All patients underwent endoscopy before or after color Doppler-ultrasonic examination, and images were interpreted independently by two endoscopists. Esophageal varices were graded according to the criteria of the Japanese Research Society for Portal Hypertension and endoscopic finding of PV hypertension[10] in straight and small-calibered varices (F1), moderately enlarged beady varices (F2), or markedly enlarged nodular or tumor-shaped varices (F3).
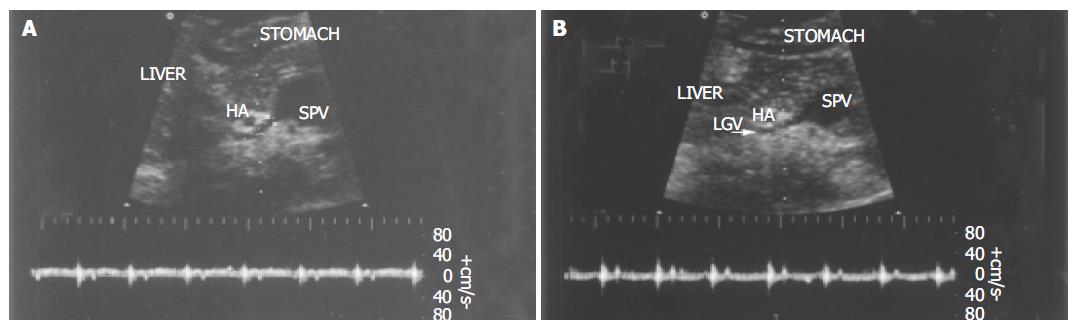
Ultrasonographic examinations were performed using HDI5000 and HPsono4500 color Doppler units with a 3.75-MHz convex probe. All the patients and controls were kept fasting overnight prior to the procedure. They were examined in the supine position during quiet respiration. Measurements of diameter, flow direction and flow velocity in the LGV and PV were done in all patients and controls. The PV blood flow was measured at the crossing point with the hepatic artery or just distally to it. The LGV usually originates from the portal-splenic vein junction or its vicinity and runs to the esophagogastric junction. It was identified longitudinally by ultrasonography in a left oblique scan in the epigastrium. Blood flow measurement was made in the straight portion of the LGV, usually within 5 cm from its origin[11,12]. The diameters of the LGV and PV were calculated from the inner surface within the vessel as seen in a longitudinal view. The sample volume was selected from 2 to 5 mm widths to include the width of the vessel. Flow direction was assessed according to the upward or downward position of the Doppler waveform over the baseline. The beam-vessel angle was less than 60° in every patient. Flow velocity was calculated as an average value of three consecutive measu-rements. The operator was blind to any information on the endoscopic findings of varices and the portal pressure.
After baseline measurements, 30 min after oral admini-stration of 75 g glucose in 225 mL, changes of the diameter, flow velocity and direction in the PV and LGV were examined in 60 patients with esophageal varices and 15 healthy controls.
Student’s t-test was used for single comparison, one-way analysis of variance for multiple comparisons. A P-value less than 0.05 was considered statistically significant. Statistical analyses were performed with the SPSS 10.0 software program.
The PV and LGV were detected successfully in 115 (96%) and 105 (88%) of 120 cirrhotic patients, respectively, and in 27 (90%) and 21 (70%) of 30 healthy volunteers, respectively. Among the 120 cirrhotic patients, 37 had F1, 59 had F2, and 24 had F3 grade varices.
Table 1 summarizes the duplex sonography findings. Compared with the healthy controls, cirrhotic group had a significantly lower velocity in the PV, a significantly greater diameter of the PV and LGV, a higher velocity in the LGV. In the cirrhotic group, no difference in portal flow velocity and diameter was observed between patients with or without EVB. However, the diameter and blood flow velocity of the LGV were significantly higher in EVB (+) group compared with EVB (-) group (P<0.01).
Diameter of the LGV increased with enlarged size of varices. In patients with F1, F2, and F3 varices, the diameter was 0.48±0.16, 0.62±0.23, and 0.72±0.24 cm, respectively. There were differences between F1 and F2, F1 and F3 varices, but no differences between F2 and F3 varices (P = 0.125). However, variceal bleeding was more frequent in patients with a diameter of the LGV >6 mm, suggesting that the diameter of LGV had a relationship with the size of esophageal varices, but had no value in the identification of patients with cirrhosis at risk for EVB (Table 2).
| Varices | Diameter (cm) | Velocity (cm/s) | ||
| PV | LGV | PV | LGV | |
| F1 | 1.47±0.19 | 0.48±0.16 | 14.2±2.15 | 7.80±2.15 |
| F2 | 1.51±0.18 | 0.62±0.23 | 13.1±1.81 | 11.5±2.03 |
| F3 | 1.55±0.21 | 0.72±0.24 | 12.0±1.72 | 16.0±3.19 |
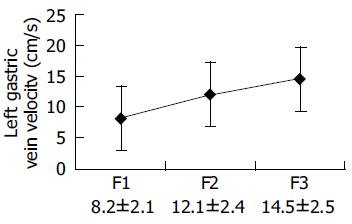
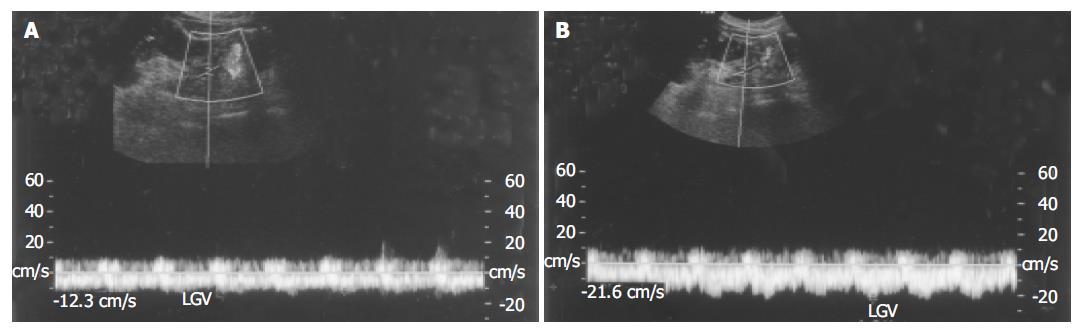
The flow velocity in LGV, in healthy controls, was 8.70±1.91 cm/s (n = 21). In patients with liver cirrhosis, it was 10.3±2.1 cm/s (n = 12) when the flow was hepatopetal and 13.5±2.3 cm/s (n = 87) when the flow was hepatofugal (Figure 1). As the size of varices enlarged, hepatofugal flow velocity increased (P<0.01) and was significantly different between patients with F1 and F2 varices, and between patients with F2 and F3 varices. Variceal bleeding was more frequent in patients with a hepatofugal flow velocity >15 cm/s (32 of 52 patients, 61.5%). Within the bleeding group, the mean LGV blood flow velocity was 16.6±2.62 cm/s. No correlation was observed between the portal blood flow velocity and EVB. Hepatofugal flow velocity of the LGV in relation to the development of esophageal varices is shown in Figure 2.
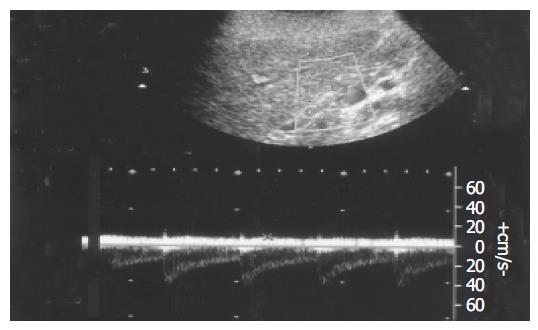
In all healthy controls, the flow direction in the LGV was hepatopetal, toward the PV (Figure 3). In patients with F1 varices, flow direction was hepatopetal in 10 patients, to-and-fro state in 3 patients, and hepatofugal in the remaining 18. The flow direction was hepatofugal in 91% patients with F2 and all F3 varices (Table 3).
| Varices | n | Flow direction | ||
| Hepatopetal | Hepatofugal | To-and-fro | ||
| F1 | 31 | 10 (32) | 18 (58) | 3 (10) |
| F2 | 53 | 2 (4) | 48 (91) | 3 (5) |
| F3 | 21 | 0 | 21 (100) | 0 |
| Sum | 105 | 12 (11) | 87 (83) | 6 (6) |
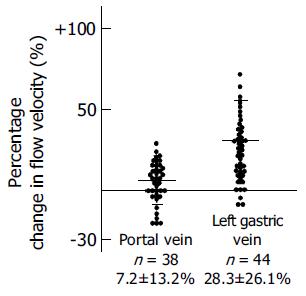
Changes in diameter of the PV and LGV were not significant before and after ingestion of glucose (PV: 1.41±1.5 cm before and 1.46±1.6 cm after; LGV: 0.57±1.7 cm before and 0.60±1.5 cm after). Flow direction in the LGV was hepatopetal and to-and-fro in 16 patients and hepatofugal in 44 patients before ingestion of glucose. Flow direction changed to hepatofugal in 9 of 16 patients with hepatopetal and to-and-fro blood flow after ingestion of glucose (Figure 4). In 44 patients with hepatofugal blood flow in the LGV, a significant increase in hepatofugal flow velocity was observed in 38 of 44 patients (86%) with esophageal varices (Figure 5). The changes of blood flow velocity were greater in the LGV than in the PV (LGV: 28.3±26.1%, PV: 7.2±13.2%, P<0.01). The degree of changes in percentages in the LGV and PV of patients with hepatofugal flow after ingestion of glucose is shown in Figure 6. There was a relationship between the percentage changes in flow velocity and the size of varices. Patients who responded excessively to food ingestion might have a high risk for bleeding, whereas no significant changes occurred in the LGV before and after ingestion of glucose in the control subjects.
EVB is a potentially deadly complication in patients with liver cirrhosis and portal hypertension. Once esophageal varices occur, the risk for EVB ranges from 10% to 60%, and the mortality rate related to EVB ranges from 20% to 60%[1-4]. Since esophageal varices-induced hemorrhage in patients with cirrhosis can be fatal, these patients must be routinely classified according to their risk status and appropriate prophylactic measures should be taken to prevent hemorrhage. The size of esophageal varices is one of the strongest risk factors for variceal rupture[1-5]. Differences in connecting venous structures and their underlying hemodynamics may be predisposing factors in the progression of esophageal varices. The ultrasonographic examination is a simple, inexpensive, accurate, and noninvasive technique to evaluate the hemodynamics under physiological conditions and has been widely used experimentally and clinically, but there is a continuing debate concerning the hemodynamics of the PV system in relation to the development of esophageal varices[6-9].
The notable findings of this study are related to the hemodynamics of the PV. Compared with the healthy controls, cirrhotic group had a significantly lower velocity in the PV and a significantly greater diameter of the PV. These findings indicate that the portal venous system in cirrhotic patients with portal hypertension is the site of passive congestion and increased blood flow. During the period of 2 years, 52 (43%) patients had at least one episode of acute esophageal variceal hemorrhage. The portal hemodynamic features had no differences between the EVB (+) and EVB (-) subgroups. It is generally thought that elevated vascular resistance and increased portal blood inflow are the two principal mechanisms involved in the development of portal hypertension secondary to cirrhosis[13]. The former is an initiating factor, while the latter plays an important role in maintaining a chronic portal hypertensive state. Therefore, portal flow velocity is unrelated to the degree of endoscopic abnormalities in patients with liver cirrhosis and has no value in the identification of patients with cirrhosis at risk for EVB. Therefore, passive congestion of portal blood has no direct relationship with EVB.
In cirrhotic patients, because of portal outflow obstruction (i.e., elevated intrahepatic portal vascular resistance), increased blood flow in the splenic vein cannot enter the liver via the PV, and a considerable percentage of splenic vein flow is forced to bypass the liver. One of the most important shunting routes is the LGV, which may normally arise from the PV and splenic vein. When increased flow in the splenic vein is prominent, the diversion of a large quantity of portal flow via the LGV would result in more severe esophageal varices and might trigger the occurrence of EVB[14,15].
This study showed a close relationship between the velocity of hepatofugal flow in the LGV and esophageal variceal size. EVB was more frequent in patients with a hepatofugal flow velocity >15 cm/s (32 of 52 patients, 61.5%). Preservation of hepatopetal flow in the LGV in patients with portal hypertension may be associated with a low risk for variceal hemorrhage. Because the LGV is the major source of blood supply to esophageal varices, velocity of hepatofugal flow may be the more important determinant in the development of varices. These results are similar to those of previous studies[16-18]. Therefore, detection of a high-flow velocity in the LGV may be suggestive of hemodynamically active esophageal varices that carry a high risk for bleeding.
The diameter of the LGV increased as the size of varices enlarged, there were differences between patients with F1 and F2, F1 and F3 varices, but no differences between patients with F2 and F3 varices, suggesting that diameter of the LGV has a relationship with the size of esophageal varices, but has no value in the identification of patients with cirrhosis at risk for EVB. The diameter of the LGV may measure up to 6 mm on sonograms of normal subjects. However, variceal bleeding is more frequent in patients with a diameter of the LGV >6 mm.
It is generally thought that flow direction in the LGV changes from hepatopetal to hepatofugal during variceal development[17,18]. However, in our study the flow direction in the LGV was still hepatopetal and LGV did not seem to contribute to the formation of esophageal varices in 32% patients with F1 varices. Blood flow in the stomach wall from the left gastric artery also participates in variceal blood flow[19-21]. These blood flows in the gastric wall alone possibly contribute to the formation of esophageal varices in its early stage, where LGV flow does not significantly constitute to the variceal flow. In advanced portal hypertension, esophageal varices become large as the LGV flow changes to hepatofugal and drains into the varices. Whereas a to-and-fro flow state is generally considered to be present in the LGV during the period when the flow becomes reversed[19], which was observed in six patients in the present study. Other studies reported that there is a relationship between esophageal varices and venous collaterals outside the esophageal wall in patients with portal hypertension. Collaterals are divided into periesophageal collateral veins and paraesophageal collateral veins. Periesophageal collateral veins play a more important role in the formation of esophageal varices than paraesophageal collateral veins in the early stage of esophageal varices[21-24].
Food intake usually increases portal blood flow. In this study, we gave a dose of glucose and an increase of hepatofugal blood flow velocity was observed in the LGV and PV. Also the changes of blood flow velocity were greater in the LGV than in the PV. Out of 44 patients with hepatofugal blood flow in the LGV, a significant increase in hepatofugal flow velocity was observed in 38 patients (86%) with esophageal varices. There was a relationship between the percentage changes in flow velocity and the size of varices. Patients who responded excessively to food ingestion might have a high risk for bleeding. High flow resistance in a portal hypertensive liver may exaggerate the increase in intestinal inflow into the LGV, henceforth into the esophageal varices under such conditions[17], because blood flow in the LGV tends to increase readily when the portal blood flow increases or portal flow resistance is elevated. In patients with portal hypertension, an increase in variceal blood flow may contribute to bleeding from esophageal varices. The analysis of these factors influencing blood flow in esophageal varices seems to be important to understand the pathophysiology of variceal bleeding and helps us to estimate the risk for variceal hemorrhage[25-28].
In conclusion, PV is unrelated to the degree of endoscopic abnormalities in patients with liver cirrhosis and its measu-rement by Duplex-Doppler ultrasonography has no value in the identification of patients with cirrhosis at risk for variceal bleeding. Hemodynamics of the LGV appears to be superior to those of the PV in predicting bleeding, allowing physicians to optimize therapy[29]. Therefore, the likelihood index, adopted to determine the best parameters related to variceal bleeding showed that the most important combinations are endoscopic findings followed by the LGV hemodynamics.
Co-first-authors: Feng-Hua Li and Jing Hao
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Brandenburger LA, Regenstein FG. Variceal Hemorrhage. Curr Treat Options Gastroenterol. 2002;5:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Seewald S, Seitz U, Yang AM, Soehendra N. Variceal bleeding and portal hypertension: still a therapeutic challenge? Endoscopy. 2001;33:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Bhasin DK, Malhi NJ. Variceal bleeding and portal hypertension: much to learn, much to explore. Endoscopy. 2002;34:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Tsokos M, Türk EE. Esophageal variceal hemorrhage presenting as sudden death in outpatients. Arch Pathol Lab Med. 2002;126:1197-1200. [PubMed] |
| 6. | Martins RD, Szejnfeld J, Lima FG, Ferrari AP. Endoscopic, ultrasonographic, and US-Doppler parameters as indicators of variceal bleeding in patients with schistosomiasis. Dig Dis Sci. 2000;45:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Erdozain Sosa JC, Martín Hervás C, Moreno Blanco MA, Zapata Aparicio I, Herrera Abián A, Conde Gacho P, Madero R, Segura Cabral JM. [Color duplex Doppler ultrasonography in the evaluation of the risk of esophageal varices bleeding in cirrhotic patients]. Gastroenterol Hepatol. 2000;23:466-469. [PubMed] |
| 8. | Piscaglia F, Donati G, Serra C, Muratori R, Solmi L, Gaiani S, Gramantieri L, Bolondi L. Value of splanchnic Doppler ultrasound in the diagnosis of portal hypertension. Ultrasound Med Biol. 2001;27:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Cioni G, Tincani E, Cristani A, Ventura P, D'Alimonte P, Sardini C, Turrini F, Abbati GL, Romagnoli R, Ventura E. Does the measurement of portal flow velocity have any value in the identification of patients with cirrhosis at risk of digestive bleeding? Liver. 1996;16:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Liang ZH, Li shb. portal vein hypertension. publishing company of people's military Dr. . |
| 11. | Roi DJ. Ultrasound anatomy of the left gastric vein. Clin Radiol. 1993;47:396-398. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Xia JG, Dong SQ, Li FH. Sonography and hemodynamic features of normal left gastric vein. Shijie Huaren Xiaohua Zazhi. 2003;11:491-493. |
| 13. | Benoit JN, Womack WA, Hernandez L, Granger DN. "Forward" and "backward" flow mechanisms of portal hypertension. Relative contributions in the rat model of portal vein stenosis. Gastroenterology. 1985;89:1092-1096. [PubMed] |
| 14. | Kotenko OG. The state of regional hemodynamics after performance of operations of the portogastroesophageal blood flow disconnection in liver cirrhosis. Klin Khir. 2000;7:19-21. [PubMed] |
| 15. | Nakano R, Iwao T, Oho K, Toyonaga A, Tanikawa K. Splanchnic hemodynamic pattern and liver function in patients with cirrhosis and esophageal or gastric varices. Am J Gastroenterol. 1997;92:2085-2089. [PubMed] |
| 16. | Wachsberg RH, Simmons MZ. Coronary vein diameter and flow direction in patients with portal hypertension: evaluation with duplex sonography and correlation with variceal bleeding. AJR Am J Roentgenol. 1994;162:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hino S, Kakutani H, Ikeda K, Uchiyama Y, Sumiyama K, Kuramochi A, Kitamura Y, Matsuda K, Arakawa H, Kawamura M. Hemodynamic assessment of the left gastric vein in patients with esophageal varices with color Doppler EUS: factors affecting development of esophageal varices. Gastrointest Endosc. 2002;55:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Matsutani S, Furuse J, Ishii H, Mizumoto H, Kimura K, Ohto M. Hemodynamics of the left gastric vein in portal hypertension. Gastroenterology. 1993;105:513-518. [PubMed] |
| 19. | Irisawa A, Shibukawa G, Obara K, Saito A, Takagi T, Shishido H, Odajima H, Abe M, Sugino T, Suzuki T. Collateral vessels around the esophageal wall in patients with portal hypertension: comparison of EUS imaging and microscopic findings at autopsy. Gastrointest Endosc. 2002;56:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Widrich WC, Srinivasan M, Semine MC, Robbins AH. Collateral pathways of the left gastric vein in portal hypertension. AJR Am J Roentgenol. 1984;142:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Irisawa A, Obara K, Sato Y, Saito A, Takiguchi F, Shishido H, Sakamoto H, Kasukawa R. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointest Endosc. 1999;50:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Escorsell A, Garcia-Pagán JC, Bosch J. Assessment of portal hypertension in humans. Clin Liver Dis. 2001;5:575-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bolognesi M, Sacerdoti D, Merkel C, Bombonato G, Gatta A. Noninvasive grading of the severity of portal hypertension in cirrhotic patients by echo-color-Doppler. Ultrasound Med Biol. 2001;27:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Yin XY, Lu MD, Huang JF, Xie XY, Liang LJ. Color Doppler velocity profile assessment of portal hemodynamics in cirrhotic patients with portal hypertension: correlation with esophageal variceal bleeding. J Clin Ultrasound. 2001;29:7-13. [PubMed] [DOI] [Full Text] |
| 25. | Matsutani S, Maruyama H, Sato G, Fukuzawa T, Mizumoto H, Saisho H. Hemodynamic response of the left gastric vein to glucagon in patients with portal hypertension and esophageal varices. Ultrasound Med Biol. 2003;29:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Silva G, Navasa M, Bosch J, Chesta J, Pilar Pizcueta M, Casamitjana R, Rivera F, Rodés J. Hemodynamic effects of glucagon in portal hypertension. Hepatology. 1990;11:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Okazaki K, Miyazaki M, Onishi S, Ito K. Effects of food intake and various extrinsic hormones on portal blood flow in patients with liver cirrhosis demonstrated by pulsed Doppler with the Octoson. Scand J Gastroenterol. 1986;21:1029-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Lin HC, Yang MC, Hou MC, Lee FY, Huang YT, Lin LF, Li SM, Hwang SJ, Wang SS, Tsai YT. Hyperglucagonaemia in cirrhotic patients and its relationship to the severity of cirrhosis and haemodynamic values. J Gastroenterol Hepatol. 1996;11:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Schepis F, Cammà C, Niceforo D, Magnano A, Pallio S, Cinquegrani M, D'amico G, Pasta L, Craxì A, Saitta A. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |