Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4547
Revised: December 15, 2004
Accepted: December 21, 2004
Published online: August 7, 2005
AIM: To investigate the effect of release behavior of sustained-release dosage forms of sinomenine hydrochloride (SM•HCl) on its pharmacokinetics in beagle dogs.
METHODS: The in vitro release behavior of two SM•HCl dosage forms, including commercial 12-h sustained-release tablets and 24-h sustained-release pellets prepared in our laboratory, was examined. The two dosage forms were orally administrated to beagle dogs, and then the in vivo SM•HCl pharmacokinetics was investigated and compared.
RESULTS: The optimal SM•HCl sustained-release formulation was achieved by mixing slow- and rapid-release pellets (9:1, w/w). The SM•HCl release profiles of the sustained-release pellets were scarcely influenced by the pH of the dissolution medium. Release from the 12-h sustained-release tablets was markedly quicker than that from the 24-h sustained-release pellets, the cumulative release up to 12-h was 99.9% vs 68.7%. From a pharmacokinetic standpoint, the 24-h SM•HCl sustained-release pellets had longer tmax and lower Cmax compared to the 12-h sustained-release tablets, the tmax being 2.67×0.52 h vs 9.83×0.98 h and the Cmax being 1 334.45±368.76 ng/mL vs 893.12±292.55 ng/mL, respectively. However, the AUC0-tn of two SM•HCl dosage forms was comparable and both preparations were statistically bioequivalent. Furthermore, the two preparations had good correlations between SM•HCl percentage absorption in vivo and the cumulative percentage release in vitro.
CONCLUSION: The in vitro release properties of the dosage forms strongly affect their pharmacokinetic behavior in vivo. Therefore, managing the in vitro release behavior of dosage forms is a promising strategy for obtaining the optimal in vivo pharmacokinetic characteristics and safe therapeutic drug concentration-time curves.
- Citation: Sun J, Shi JM, Zhang TH, Gao K, Mao JJ, Li B, Sun YH, He ZG. Impact of release characteristics of sinomenine hydrochloride dosage forms on its pharmacokinetics in beagle dogs. World J Gastroenterol 2005; 11(29): 4547-4551
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4547.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4547
Sinomenine [(9α, 13α, 14α)-7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methyl- morphinan-6-one] is an active alkaloid, which can be extracted from the stems of Sinomenium acutum Rehd. et Wils. Generally, sinomenine hydrochloride (SM•HCl) is the main chemical form for pharmaceutical purposes. Recent studies have shown that SM•HCl possesses potent anti-inflammatory, analgesic, and immunoinhibitory pharmacological effects, which provide the basis for the treatment of rheumatoid arthritis. Many clinical therapeutical trials have confirmed the effectiveness of SM•HCl in treating rheumatoid arthritis and the efficacy is as high as 90%[1].
The conventional SM•HCl dosage forms (injection and rapid-release tablets) require frequent administration (3-4 times/d) and even the sustained-release tablets available on the market require administration twice per day, thus leading to poor patient compliance. Additionally, the high fluctuation in the SM•HCl plasma levels during multi-dose therapy with the marketed SM•HCl dosage forms contributes to side effects, such as gastrointestinal tract toxicity and allergic reactions[2]. Accordingly, it is necessary to develop 24-h SM•HCl sustained-release dosage forms (for once-daily administration) in order to reduce side effects and increase patient compliance.
Currently, many controlled/sustained-release dosage forms are being converted from single unit drug delivery systems (DDS) such as tablets to multiple unit DDS such as pellets for the following reasons. Firstly, pellets seem to be less influenced by physiologic factors such as gastric emptying and intestinal transit than tablets, resulting in less marked inter-individual differences. Secondly, pellets are widely and evenly distributed over the surface of the gastrointestinal tract, increasing drug-gastrointestinal contact, thus improving oral bioavailability. Thirdly, release failures of individual units hardly affect the total release behavior due to the presence of multiple units, unlike the case with tablets[3]. Finally, optimal release characteristics can be achieved using a mixing strategy involving pellets with different release properties[3].
For these reasons, we decided to design SM•HCl sustained-release pellets, and to manage the in vitro drug release in a manner that would allow 24-h sustained release. Furthermore, the in vivo pharmacokinetic properties of the 24-h sustained-release pellets were compared with those of the marketed 12-h sustained-release tablets in beagle dogs.
SM•HCl was obtained from Hunan Zhengqing Pharm. Co. (Changsha, China). Microcrystalline cellulose (MCC, Avicel PH101) was provided by Changshu Pharm. Adjunct Co. (Changshu, China). Eudragit NE 30D was provided by Röhm Pharma GmbH (Darmstadt, Germany). Reference formulation: SM•HCl sustained-release tablets (Zhengqing Pharm. Co., each tablet containing 60 mg of SM•HCl); Test formulation: SM•HCl 24-h sustained-release pellets in capsules (each containing 120 mg).
Preparation of blank core pellets Blank core pellets (0.8-1.1 mm in diameter) were prepared using MCC as a matrix and water as the adhesive by a rotary layering process (BZJ-360IIM rotary processor, 15th Institute of Academy for Chinese Carrier-Rocket Technology, Beijing). Four hundred grams of MCC was placed in the rotary processor chamber. The inlet airflow was 10-20 L/min, the atomizing pressure was 0.1-0.3 MPa and the rotation speed was 200 r/min. Then, the adhesive was sprayed at a flow rate of 15-25 r/min. Finally, the products were dried at 60 °C and then sieved to obtain blank core pellets of the required size.
Preparation of SM•HCl pellets Four hundred grams of blank core pellets were placed in the rotary processor chamber at a rotation speed of 200 r/min. SM•HCl in the drug supply container was added to chamber at 10-20 r/min. One percent of hydroxypropylmethyl cellulose (5 cps) in water was used as an adhesive, and sprayed over the pellets at a rate of 10-15 r/min. Finally, the products were dried at room temperature[4,5].
Preparation of SM•HCl 24-h sustained-release pellets The pellets were coated in a fluidized-bed coating apparatus (Shenyang Pharmaceutical University, China). For the coating process, the nozzle port size was 1 mm, the inlet air temperature was 18-23 °C, the atomizing pressure was 1.0 kg/cm2, and the coating solution was sprayed onto the pellets at a flow rate of 0.8-1.2 mL/min. The coating solution was an aqueous dispersion of Eudragit NE 30D. Talc- an antiadherent agent and sodium dodecyl sulfate- an anti-static agent, were added to the coating solution. Finally, the coated pellets were cured by heating at 40 °C for 24 h[6,7].
Dissolution test Dissolution studies were carried out using the basket method at a rotation speed of 100 r/min at 37 °C in 900 mL dissolution medium according to ChP 2000. The dissolution tests were performed in 0.1 HCl, pH 6.8 or 7.4 PBS and distilled water, respectively. Samples were collected at predetermined time points, and the SM•HCl content was analyzed using a UV-spectrophotometer (UV-9100, Ruili Co., China) at 365 nm. Then cumulative percentage of SM•HCl release was calculated.
Experimental protocol All animal studies were performed according to the Guidelines for the Care and Use of Laboratory Animals approved by the Ethics Committee of Animal Experimentation of Shenyang Pharmaceutical University. Six male beagle dogs (weighing 202.5 kg) were randomly assigned to one of two crossover experiments with a 7-d washout period. Dogs were fasted for 12 h before administration with free access to water. Each dog was given orally either reference (two sustained-release tablets) or test formulation (one capsule of sustained-release pellets). Blood samples were collected at predetermined times for each protocol: (1) 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 14, and 24 h for the reference; (2) 0, 1, 2, 4, 6, 8, 9, 10, 11, 12, 14, 24, and 36 h for the test. Plasma was immediately obtained by centrifuging blood samples at 3 000 r/min for 10 min. The plasma samples were stored in a freezer at -20 °C until analysis.
Chromatographic conditions Quantitative determination was performed on a high-performance liquid chromatograph (HPLC) equipped with a PU-980 pump (Jasco, Japan) and UV-975 detector (Jasco). An ODS C-18 (4.6 mm200 mm, 5 μm) was used and the mobile phase consisted of methanol:acetonitrile:0.3% PBS (pH 4.8, 240:80:1 180, v/v)[7]. The eluates were monitored at 265 nm. The flow rate was 0.9 mL/min and the column temperature was maintained at 30 °C.
Sample preparation Each plasma sample (0.5 mL) was mixed with 100 μL of 1.5 μg/mL caffeine and 200 μL amine-ammonium chloride aqueous solution (pH 11) in a glass centrifuge tube, followed by the addition of 3 mL of a solution of hexane:dichloride methane:isopropyl alcohol (100:50:5, v/v). The mixture was then shaken on a vortex mixer for 3 min and centrifuged at 3 000 r/min for 10 min. The organic layer was transferred to a clean tube and evaporated to dryness under nitrogen at 50 °C. The residue was dissolved in 100 μL of 0.5% phosphoric acid and 20 μL was subjected to HPLC.
Data analysis The term tmax denotes the time to reach peak concentration, and Cmax is the peak concentration, and they were obtained directly from the measured values. The elimination rate constant (Ke) was calculated from the slope of the logarithm of the plasma concentration vs time using the final four points. The parameter t0.5 was derived from 0.693/Ke. The area under the plasma concentration-time curve (AUC0-tn) until the last sampling time (tn) was calculated by the trapezoidal method. The relative bioavailability (F%) was calculated as AUCTest formulation/AUCReference formulation. The percentage absorption in vivo was calculated by Wagner-Nelson method.
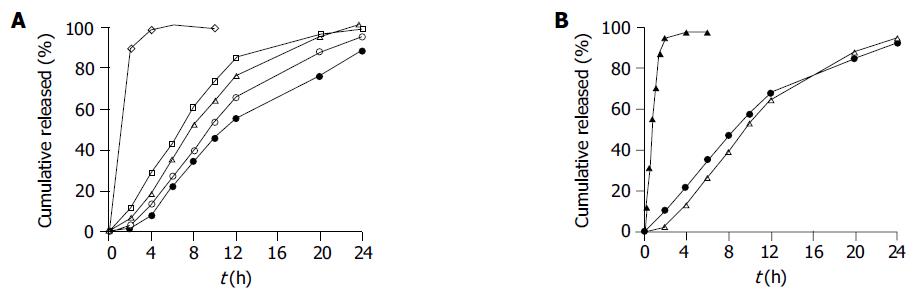
The SM•HCl-loaded pellets were coated using the coating formulation consisting of 117 g/L Eudragit NE 30D, sodium dodecyl sulfate (1% of Eudragit NE 30D, w/w) as an antistatic agent and talc (45% of Eudragit NE 30D, w/w) as an antiadherent agent in aqueous dispersion. The effect of the coating level on the in vitro release behavior of SM•HCl sustained-release pellets is shown in Figure 1A. Clearly, higher the coating level, slower the release of SM•HCl from the coated pellets. Judged from the initial release rate and cumulative percentage release up to 20 h, the ideal release behavior was achieved at a coating level of 6.5%. However, drug release was still rather slow in the initial phase, with 2.84% being released up to 2 h. Therefore, SM•HCl rapid-release pellets were prepared with a coating level of 0.1%, and their release profiles are shown in Figure 1B, and almost complete release was obtained within 2 h. When employing a 9:1 (w/w) mixture ratio of slow- and rapid-release pellets, the SM•HCl cumulative release was 10% at 2 h, 48% at 8 h, and more than 85% up to 20 h (Figure 1B). This formulation was regarded as the optimal sustained-release formulation for the following studies.
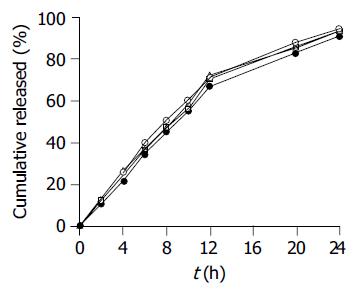
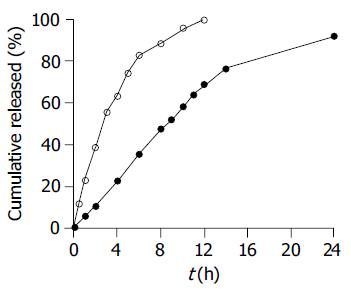
The effect of the pH of the dissolution medium on the release profiles of SM•HCl sustained-release pellets is shown in Figure 2. The pH had no significant effect on the SM•HCl release properties of the coated pellets. The release profiles of the reference 12-h and test 24-h sustained-release tablets and pellets were compared (Figure 3). The reference formulation released drug completely up to 12 h, while the test formulation only released 68% up to 12 h with near complete release until 24 h.
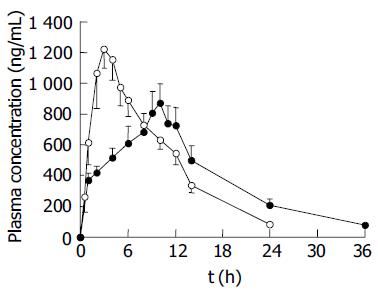
Either SM•HCl reference formulation (two 60 mg 12-h sustained-release tablets) or SM•HCl test formulation (one capsule containing 120 mg 24-h sustained-release pellets) was orally administered in the two crossover experiments in beagle dogs. The concentration-time curves of SM•HCl in plasma for the two dosage forms are illustrated in Figure 4. The pharmacokinetic parameters were calculated (Table 1). The tmax was 2.67±0.21 and 9.83±0.40 h for SM•HCl reference and test formulations, respectively, indicating that the test pellets underwent a slower release and prolonged absorption than the reference tablets. Also, the Cmax of SM•HCl 24-h sustained-release pellets was 893.1±119.4 ng/mL, which was significantly lower than that of 12-h sustained-release tablets (1 334.5±150.5 ng/mL, P<0.05). Furthermore, the AUC0-36 of the SM•HCl test formulation (13.50±1.47 mg•h/L) was similar to the AUC0-24 of the SM•HCl reference formulation (13.06±9.45 mg·h/L), with the F (%) being 103.4. Hence, the two formulations were statistically bioequivalent (P<0.05).
| Parameter | Test formulation | Reference formulation |
| Cmax (ng/mL) | 893.1±119.4 | 1 334.5±150.5 |
| tmax (h) | 9.83±0.40 | 2.67±0.21 |
| Ke (1/h) | 0.044±0.01 | 0.084±0.01 |
| t0.5 (h) | 20.7±8.7 | 8.6±1.9 |
| AUC0–tn (mg·h/L) | 13.50±1.47 | 13.06±9.45 |
| F (%) | 103.41 | - |
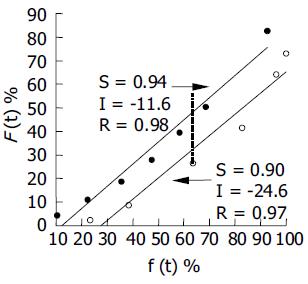
The percentage absorption in vivo of the two formulations was assessed by the Wagner-Nelson method. The percentage absorption (F(t)) exhibited a good linear relationship with the cumulative percentage release (f(t), r being 0.98 and 0.97 for the test and reference formulations, respectively, Figure 5), demonstrating a good correlation between the in vitro release and in vivo absorption processes.
It has been well established that SM•HCl is in an effective treatment for rheumatoid arthritis comparable with another traditional Chinese medicine, total glycosides of tripterygium Wilfordii Hook f. However, SM•HCl has markedly reduced side effects and improved safety, compared to the latter in terms of gastrointestinal tract effects and urogenital toxicity[8]. In spite of this, the conventional SM•HCl dosage forms give rise to higher peak to trough plasma concentration fluctuation, resulting in moderate side effects. For this reason, we attempted to develop a 24-h SM•HCl sustained-release preparation (once daily), and to prolong the absorption phase and reduce the degree of concentration fluctuation in vivo by managing the drug release characteristics of dosage forms in vitro.
The multiple unit drug DDS and SM•HCl pellets were prepared by a rotary layering process and the sustained-release behavior was obtained by standard industrial coating technology. The coating formulation consisted of an aqueous dispersion of Eudragit NE 30D[9], with the addition of talc as the antiadherent agent and sodium dodecyl sulfate as the antistatic agent. The coating layer of Eudragit NE 30D was moderately permeable to water molecules, which are capable of penetrating into the pellet core. Thus, a saturable drug solution was formed within the Eudragit NE 30D coating layer and provided the primary drive for drug release. Two mechanisms were assumed to account for drug release from the coated pellets. Provided the coating layer was a homogenous uninterrupted membrane, the additives were evenly distributed, and there were many interstices of the correct molecular size in the membrane. Drug molecules initially dissolved in the membrane, then diffused down the concentration gradient through these micro-gaps, and finally released into the outer medium. In addition, if a water-soluble channeling agent was added, a number of aqueous micro-channels formed across the membrane when coming into contact with water, and acted as release paths for soluble drug molecules, such as SM•HCl[10].
In this study, when a small amount of channeling agent (1% PEG 4000 of Eudragit NE 30D, w/w) was added to the coating formulation, SM•HCl-coated pellets at the coating level of 6.5% released drug completely up to 12 h (unpublished data). Thus, the channeling agent was absent in the coating formulation, and the former mechanism is responsible for water-soluble SM•HCl release from the coated pellets. Its release behavior corresponds to Fick’s diffusion equation: dC/dt = -DAk△C/h, where dC/dt is the diffusion rate, D the diffusion coefficient, A the surface area of membrane, k the partition coefficient, △C the concentration difference across membrane, and h the thickness of membrane. Since D, k and A were given for SM•HCl and coating membrane, and △C was constant in the initial release phase and decreased exponentially in the later phase, dC/dt was inversely related to h, as evidenced in Figure 1A. When the 6.5% coating level was used, ideal release behavior was achieved. Unfortunately, it exhibited a rather slower release in the initial phase up to 2 h. With regard to mixing pellets with different release rates to obtain the desired release behavior, rapid-release pellets with complete release within 2 h were prepared and combined with the above slow-release pellets (1:9, w/w). The final product exhibited optimal release behavior with 10.5% being released up to 2 h (Figure 1B), indicating that mixing pellets with different release rates is a powerful strategy for the management of in vitro drug release properties[11].
The release profile of the final SM•HCl sustained-release pellets was unaffected by the pH of dissolution medium (Figure 2). This was to be expected because the matrix (Eudragit NE 30D) of the membrane was insensitive to pH and the drug release was unaffected by the pH. The 24-h SM•HCl sustained-release pellets maintained a zero-rate release up to 14 h, and exhibited prolonged release up to 24 h, in contrast to the 12-h SM•HCl sustained-release tablets (Figure 3). In order to investigate the effect of in vitro release behavior on in vivo absorption of the coated pellets, an in vivo comparative pharmacokinetic evaluation was undertaken using 24-h SM•HCl sustained-release pellets vs marketed 12-h sustained-release tablets in beagle dogs.
After oral administration at a single dose of 120 mg to beagle dogs, the tmax of the 12-h sustained-release tablets was 2.670.52 h, 7 h faster than 9.830.98 h for the 24-h sustained-release pellets (Table 1 and Figure 4). In addition, the Cmax of the 12-h sustained-release tablets (1 334.45 368.76 ng/mL) was significantly higher than that of the 24-h sustained-release pellets (893.12292.55 ng/mL). Combining the Cmax and tmax, the 24-h test pellets exhibited more significant sustained release and prolonged absorption characteristics than the 12-h reference tablets, including a reduced peak concentration and a prolonged time to reach the peak concentration. More importantly, the t0.5 of the 24-h test pellets (20.78.7 h) was higher than that of the 12-h reference tablets (8.61.9 h). This result is obviously inconsistent with the theory that the intrinsic biologic half-time depends on a given drug while being independent of the type of dosage forms. The prolonged t0.5 of the 24-h test pellets suggested a reduced apparent Ke compared to the 12-h reference tablets (Table 1), which was accounted for by prolonged absorption to partially counteract the intrinsic elimination capability during the terminal phase. In effect, the absorption of the 24-h test pellets was markedly slower than that of the 12-h reference tablets (Figure 4), due to more prolonged drug release compared to the reference tablets. The aforementioned results clearly indicate that the release characteristics of dosage forms, play a significant role in mediating their pharmacokinetic behavior. As a consequence, an optimal pharmacokinetic performance could be achieved by producing the desired release profiles of dosage forms. The AUC0-tn of the two SM•HCl dosage forms was comparable and both were statistically bioequivalent preparations (P<0.05). Moreover, the SM•HCl percentage absorption in vivo correlated well with the cumulative percentage release in vitro as far as two preparations were concerned (Figure 5), validating the in vitro dissolution conditions and verifying the utility of the sustained-release dosage forms (Figure 4). In addition, the slopes of two correlation equations for the two dosage forms were similar. In contrast, the intercept of the 24-h test formulation (-11.6%) was greater than that of the 12-h reference tablets (-24.6%), suggesting that the percentage absorption of the 12 h reference tablets is 13% lower than that of the 24-h test formulation when the percentage release is the same. Ren et al[12] reported that the permeability of SM•HCl in jejunum and ileum is poorer than that in the colon, which might be responsible for the above relatively reduced absorption of the 12-h reference tablets due to the main site of residence being the jejunum and ileum.
In summary, 24-h SM•HCl sustained-release pellets can be successively prepared by a conventional rotary layering process and standard industrial coating technology. Optimal in vitro release properties can be achieved by a mixing strategy involving pellets with different release rates. Moreover, the SM•HCl release characteristics of the dosage forms have a marked effect on their pharmacokinetics in vivo. Accordingly, managing the in vitro release behavior of dosage forms is a promising strategy for obtaining optimal in vivo pharmacokinetic characteristics and safe therapeutic drug concentration-time curves.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Wang XH, Qiu SH, Dong SX, Chou P, Wu FC. Studies on the pharmacodynamics of Sinomenine tablets. Zhongyao Yaoli Yu Linchuang. 1997;13:23-25. |
| 2. | Liu Q, Zhou LL, Li R. Research overview of Sinomenine. Zhongcaoyao. 1997;28:247-249. |
| 3. | Fan TY, Wei SL, Yan WW, Chen DB, Li J. An investigation of pulsatile release tablets with ethylcellulose and Eudragit L as film coating materials and cross-linked polyvinylpyrrolidone in the core tablets. J Control Release. 2001;77:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Vertommen J, Rombaut P, Michoel A, Kinget R. Estimation of the amount of water removed by gap and atomization air streams during pelletization in a rotary processor. Pharm Dev Technol. 1998;3:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Liew CV, Wan LS, Heng PW. Role of base plate rotational speed in controlling spheroid size distribution and minimizing oversize particle formation during spheroid production by rotary processing. Drug Dev Ind Pharm. 2000;26:953-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends used for the coating of multiparticulates: comparison of aqueous and organic coating techniques. Pharm Res. 2004;21:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Bataille B, Rahman L, Jacob M. [Parameters for the formulation of physical-technical characteristics of granules of theophylline obtained by extrusion-spheronization]. Pharm Acta Helv. 1991;66:233-236. [PubMed] |
| 8. | Sun X, Zhang SM, Tian CH, Yan L, Wang LM, Li SL. Safety of tripterygium Wilfordii. Zhongguo Xinyao Zazi. 2001;10:539-533. |
| 9. | Vecchio C, Fabiani F, Sangalli ME, Zema L, Gazzaniga A. Rotary tangential spray technique for aqueous film coating of indobufen pellets. Drug Dev Ind Pharm. 1998;24:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Radtke G, Knop K, Lippold BC. Manufacture of slow-release matrix granules by wet granulation with an aqueous dispersion of quaternary poly(meth)acrylates in the fluidized bed. Drug Dev Ind Pharm. 2002;28:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lippold BC, Monells Pagés R. Film formation, reproducibility of production and curing with respect to release stability of functional coatings from aqueous polymer dispersions. Pharmazie. 2001;56:5-17. [PubMed] |
| 12. | Ren FZ, Sun SY, Jing QF. Study of the absorption character of sinomenine in intestines. Shenyang Yaoke Daxue Xuebao. 2002;19; 165-167. |