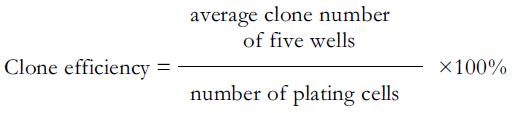Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4461
Revised: June 20, 2004
Accepted: June 24, 2004
Published online: August 7, 2005
AIM: To explore the growth inhibition and apoptosis-inducing effect of apigenin on human gastric carcinoma SGC-7901 cells.
METHODS: The effects of apigenin on the growth, clone formation and proliferation of human gastric carcinoma SGC-7901 cells were observed by MTT, clone-forming assay, and morphological observation. Fluorescent staining and flow cytometry analysis were used to detect apoptosis of cells.
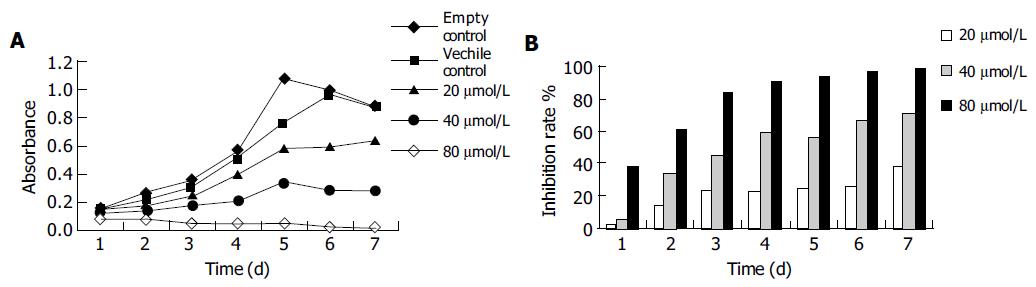
RESULTS: Apigenin obviously inhibited the growth, clone formation and proliferation of SGC-7901 cells in a dose-dependent manner. Inhibition of growth was observed on d 1 at the concentration of 80 μmol/L, while after 4 d, the inhibition rate (IR) was 90%. The growth IRs at the concentration of 20, 40, and 80 μmol/L were 38%, 71%, and 99% respectively on the 7th d. After the cells were treated with apigenin for 48 h, the number of clone-forming in control, 20, 40, and 80 μmol/L groups was 217±16.9, 170±11.1 (P<0.05), 98±11.1 (P<0.05), and 25±3.5 (P<0.05) respectively. Typical morphological changes of apoptosis was found by fluorescent staining. The cell nuclei had lost its smooth boundaries, chromatin was condensed, and cell nuclei were broken. Flow cytometry detected typical apoptosis peak. After the cells were treated with apigenin for 48 h, the apoptosis rates were 5.76%, 19.17%, and 29.30% respectively in 20, 40, and 80 μmol/L groups.
CONCLUSION: Apigenin shows obvious inhibition on the growth and clone formation of SGC-7901 cells by inducing apoptosis.
- Citation: Wu K, Yuan LH, Xia W. Inhibitory effects of apigenin on the growth of gastric carcinoma SGC-7901 cells. World J Gastroenterol 2005; 11(29): 4461-4464
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4461.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4461
Apigenin (4’, 5, 7-trihydroxyflavone), a phytopolyphenol, is widely distributed in vegetables and fruits such as celery, onion, apple, orange, etc. Recent studies have shown that apigenin exhibits anti-proliferation effects on several forms of cancer cells such as prostate cancer cells[1], breast cancer cells[2], leukemia cells[3], colon cancer cells[4-6], and enhances gap junctional intracellular communication changes in human liver cells[7] and induces morphological changes in some cells[8,9]. In addition, apigenin can suppress tumor-promoting effects of ultraviolet radiation on mouse skin[10]. Compared with other flavonoid substances, apigenin is characterized by low toxicity and non-mutagenesis[11]. Besides, it has other bioactivities such as anti-inflammatory[12] and anti-oxide[13] effects. Apigenin is a promising cancer inhibitor that may provide a new approach for the treatment of human cancers. In this article, we report the anti-proliferation effect and apoptosis-inducing effect of apigenin on human gastric carcinoma SGC-7901 cells.
Apigenin (95% purity), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) and 4’,6-diamidine-2’-phenylindole-dihydrochloride (DAPI) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). RPMI 1640 medium, ethylene diaminetetraacetic acid (EDTA), N-2-hydroxyethyl piperazine-N’-ethane sulfonic acid were obtained from Gibco Chemical Co. (Rockville, MD, USA).
Human gastric cancer SGC-7901 cells, obtained from Cancer Research Institute of Beijing (China), were grown as a monolayer in RPMI 1640 medium containing 1% penicillin/streptomycin, and 0.2% gentamicin sulfate supplemented with 100 mL/L fetal bovine serum (FBS) at 37 °C in a 50 mL/L CO2 humidified atmosphere. Apigenin was dissolved in dimethyl sulfoxide (DMSO) and mixed with a fresh medium to achieve the desired concentration. The final DMSO concentration in all media was 0.2%. This concentration of DMSO did not alter cell growth and cell cycle measurement when compared with the vehicle-free medium.
The effect of apigenin on the viability of cells was determined by MTT assay. Near-confluent stock cultures of cells were harvested with 0.2% EDTA and plated at a density of 2.5103/well in 96-well microtiter plates. After an overnight incubation to allow cell attachment, the medium was replaced by fresh medium containing different concentrations (0, 20, 40, and 80 μmol/L) of apigenin. Control wells received DMSO (0.2%). Each concentration of apigenin was repeated in four wells. After incubation for 24 h, one plate was assayed with a microplate reader at the wavelength of 570 nm. Before the assay, MTT (5 mg/mL in PBS) was added to each well and incubated for 4 h, then MTT solution was removed from the wells by aspiration. After careful removal of the medium, 0.1 mL of DMSO was added to each well, and the plate was shaken for 15 min. The data of 7 d were fed into the computer and the growth curve was drawn. The growth inhibition rate (IR) was calculated according to the following formula.
Math 1
The cells were plated at a density of 500/well on 24-well microtiter plates. After an overnight incubation to allow cell attachment, the medium was replaced by fresh medium containing DMSO (0.2%) at different concentrations (20, 40, and 80 μmol/L) of apigenin, with each concentration repeated in five wells. After being incubated for 24 or 48 h, the medium was replaced by fresh medium containing 10% FBS. The cells were incubated for another 7 d, then washed thrice with PBS and fixed in methanol for 15 min. The cells were stained with Giemsa stain. Then the number of clone-forming cells (>50 cells) was calculated under the microscope. The data were expressed as mean±SD. Clone-formation rate was calculated as follows:
Math 2
The cells were harvested with 0.2% EDTA and plated in 25-mL culture bottles at the density of 1105. After an overnight incubation to allow cell attachment, the medium was replaced by fresh medium containing DMSO (0.2%) at different concentrations (20, 40, and 80 μmol/L) of apigenin. Morphological change of the cells was observed microscopically and photographed at 24 and 48 h after the addition of apigenin.
Besides, the cells were exposed to various concentrations of apigenin (20, 40, and 80 μmol/L) for 48 h, then harvested with EDTA and washed twice with PBS. The cells were stained with 2 mg/L DAPI ethanol solution and incubated at 37 °C for 15 min. Morphological changes of the stained cells were observed under fluorescent microscope (300-500 nm) and photographed.
The cells (70% confluent) were treated with apigenin (40 and 80 μmol/L) for 48 h, then harvested with EDTA, washed twice with PBS, and centrifuged. The pellet was resuspended in 70% cold ethanol for 24 h at 4 °C. The cells were centrifuged at 110 r/min for 5 min, washed twice with PBS, suspended with 200 μL RNase A (20 μg/mL final concentration) and incubated at 37 °C for 30 min. The cells were chilled over ice for 10 min and stained with 800 μL propidium iodide (50 μg/mL final concentration) for 1 h and analyzed by flow cytometry.
All data were expressed as mean±SD and analyzed with SAS statistic software. P<0.05 was considered statistically significant.
Apigenin inhibits the cell proliferation. Previous studies have proved that apigenin can inhibit the growth of several kinds of cancer cells. In our study, we examined whether apigenin exerted a similar anti-proliferative effect on human gastric cancer SGC-7901 cells. As shown in Figure 1, the cells in control group entered the logarithmic growth phase on the 1st d after they were plated and reached their peak on the 6th d. While in the treatment groups, the growth of cells was inhibited in a dose- and time-dependent manner. Inhibition of growth was evident on d 1 at the concentration of 80 μmol/L, after 4 d the IR was 90%. The growth IR of 20, 40, and 80 μmol/L of apigenin was 38%, 71%, and 99% respectively on the 7th d.
As shown in Figure 2, after exposure to apigenin for 24 or 48 h, the clone formation of SGC-7901 cells was suppressed in a dose- and time-dependent manner. The cloning efficiency in 80 μmol/L was 9.8% and 5% after treatment with apigenin for 24 and 48 h, while in the control group it was 40.4% and 43.4% (Table 1).
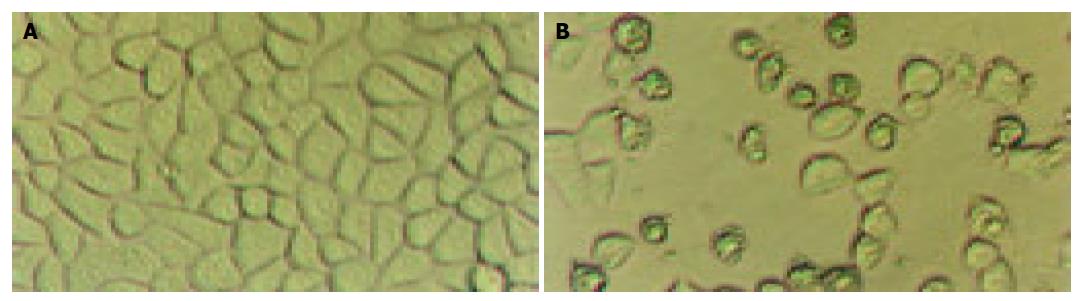
Figure 3 shows the morphological changes of SGC-7901 cells treated for 48 h with 80 μmol/L apigenin or vehicle. In the vehicle control group, DMSO (0.2%) did not induce any marked morphological change in the cells. In the DMSO group, the cells were transparent and in the great density, the boundaries of the cells were dim, the nucleolus was very clear. While in the treatment group, there was a significant decrease in quantity and transparency of the cells, the cells crimpled and the boundaries became clear, the nucleolus could not be observed clearly.
After being stained with DAPI, the cells were visualized under blue fluorescence. In the control group, the nuclei were almost round in shape with clear and smooth boundaries, the staining was equal. After treatment with apigenin for 48 h, the nuclei of cells were broken and the staining was unequal. The chromatins of cells were condensed, and the nuclei lost their smooth boundaries.
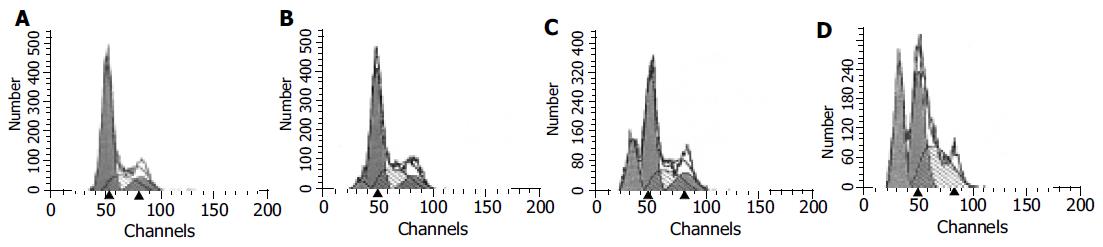
During apoptosis, the DNA is broken into small fragments and released from cells. In this experiment, apoptosis was induced by apigenin. Flow cytometry analysis results are shown in Figure 4. Apigenin (20, 40, and 80 mmol/L) treatment for 48 h induced a significant apoptosis and accumulation of cells in S phase. The apoptosis rates were 5.76%, 19.17%, and 29.30%, respectively.
The pathogenesis of cancer is a multi-phase process. Inherited and environmental factors play an important role in the occurrence of cancer. Gastric cancer is one of the most common malignant tumors in China. Some bioactive substances such as polyphonic and isoflavone exist mainly in plant-based food (fruits and vegetables). Apigenin, one of the most common flavonoids, is widely distributed in many fruits and vegetables. Studies[14] have proved that apigenin has strong anti-cancer effects.
In our experiment, we used MTT and clone-forming assay to detect the growth inhibition effect of apigenin on human gastric SGC-7901 cells. The results showed that apigenin dramatically suppressed the growth and clone formation of the cells in a dose- and time-dependent manner. After treatment of cells with 80 μmol/L apigenin for 4 d, the growth IR was above 90% and other concentrations of apigenin also suppressed cell growth to different degrees. Clone formation reflects the proliferative ability of tumor stem cells, which is the important target of anticancer treatment. Inhibition of stem cells is more effective than that of common carcinoma cells during the treatment of cancer. With fluorescence microscope, we observed typical morphological changes such as the disintegrity of nuclear membrane, condensation of chromatin and broken nuclei. FACS analysis detected special apoptosis peak, which further supports the results in fluorescence morphological observation.
In conclusion, apigenin can suppress the growth of human gastric cancer SGC-7901 cells, which is associated with its apoptosis-inducing effect.
We thank Yan Zhao, Lan Zhao and Xiao-Hua Zhang for their help and encouragement throughout the whole experiment.
Co-first-authors: Kun Wu and Lin-Hong Yuan
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413-420. [PubMed] |
| 3. | Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 310] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 752] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 1756] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 6. | Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Chaumontet C, Bex V, Gaillard-Sanchez I, Seillan-Heberden C, Suschetet M, Martel P. Apigenin and tangeretin enhance gap junctional intercellular communication in rat liver epithelial cells. Carcinogenesis. 1994;15:2325-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kuo ML, Yang NC. Reversion of v-H-ras-transformed NIH 3T3 cells by apigenin through inhibiting mitogen activated protein kinase and its downstream oncogenes. Biochem Biophys Res Commun. 1995;212:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Sato F, Matsukawa Y, Matsumoto K, Nishino H, Sakai T. Apigenin induces morphological differentiation and G2-M arrest in rat neuronal cells. Biochem Biophys Res Commun. 1994;204:578-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Lepley DM, Li B, Birt DF, Pelling JC. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 1996;17:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418-425. [PubMed] |
| 12. | Fuchs J, Milbradt R. Skin anti-inflammatory activity of apigenin-7-glucoside in rats. Res Commun Chem Pathol Pharmacol. 1989;64:69-78. |
| 13. | Lin CM, Chen CT, Lee HH, Lin JK. Prevention of cellular ROS damage by isovitexin and related flavonoids. Planta Med. 2002;68:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |