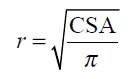INTRODUCTION
Pain arising from the esophagus is very common clinically and in the normal population, but the mechanisms involved are poorly understood[1]. Due to the difficulties in characterizing clinical pain, human experimental models have been developed to investigate the pain pathways in a standardized way in volunteers and patients. These models provide the possibility to control the stimulus parameters and to assess the response quantitatively[2,3]. Furthermore, the nociceptive system can be sensitized in the laboratory, resulting in allodynia (painful sensations to stimuli that are not normally painful), hyperalgesia (increased sensation to stimuli that are normally painful) and increase in the evoked referred pain area[4]. The sensitization most likely plays an important role in chronic visceral pain disorders[3,5]. Experimental chemical stimulation with acid has been used to sensitize the esophagus[6-8]. However, the literature has not been consistent with respect to the evoked mechanical hyperalgesia, probably due to methodological problems related to the stimulus modalities used[9].
Distension of the gut is a physiologic stimulus, and consequently most researchers have used experimental balloon distension models to investigate basic pain mechanisms in the gastrointestinal (GI) tract[10]. Most previous studies have used volume and pressure as proxies of the mechanical deformation and force applied to the gut wall[10]. However, the mechanical parameters tension, stress and strain are of more value than pressure and volume when studying the esophagus, as these parameters provide more valid information about the mechanical forces and deformation (elastic properties) during distension[11-15]. Furthermore, the muscle function is better evaluated when the forces and tensions can be quantitated, rather than measuring the luminal pressure[16-18]. However, the sensory-motor responses of the organ during a mechanical stimulus cannot be evaluated independently of the mechanical forces and deformation. Thus, phasic contractions and changes in muscle tone can influence the sensory response themselves[18], and in diseases of the esophagus hyper-reactivity may give major contribution to the symptoms[19,20]. Methods to estimate and control the mechanical response will thus allow better explanations of the effects on the sensory-motor response during the mechanical stimulations with and without sensitization of the pain system.
Systematic investigation of both the sensory and motor responses to controlled mechanical stimuli following experimental sensitization of the esophagus has to the best of our knowledge never been investigated. The aims of the current study were to (1) investigate the effect on sensitization of the esophagus with acid on the sensory response to controlled mechanical stimulation; (2) calculate the evoked referred pain areas to the mechanical stimulation before and after sensitization as a proxy for the central neuronal changes; and (3) evaluate the motor response to the sensitization by a new in vivo method evaluating the change in tension during contraction (the afterload tension) as function of the initial muscle length before the contraction (the preload radius).
MATERIALS AND METHODS
Thirty healthy subjects, 14 males and 16 females, mean age 36.5±12.9 years, were included. The subjects did not suffer from any kind of chronic pain, GI symptoms or disturbances in personality. All subjects gave informed written and verbal consent prior to the study. The protocol was approved by the local ethics committee and performed in accordance with the Helsinki Declaration.
Mechanical stimulations
The impedance planimetry system including the principle for measurement of the cross-sectional area (CSA) has been described in detail previously[18,21,22]. The 70-cm long probe with a diameter of 4.5 mm had a cylindrical large-sized bag near the tip. The bag was 40 mm in length and was made of 35-mm thick, non-conducting polyester urethane. A side-hole for acid perfusion was placed 2 cm above the bag. The probe had a four-electrode impedance planimetry system with four sets of ring electrodes inside the bag (GMC Aps, Hornslet, Denmark). The bag could be inflated with electrically conducting fluid (0.09% saline) through a pair of infusion channels. The change in impedance of the fluid during distension of the bag reflects the change in the CSA[18]. The infusion channels were connected to an infusion pump (Type 111, Ole Dich Instrument Makers, Hvidovre, Denmark) that was able to fill or empty the bag continuously. The bag could be inflated to a CSA of approximately 2 000 mm2 (diameter equal to 50 mm) without stretching the wall of the bag. The fluid in the connecting tube between the pump and the probe was heated to 37 °C. A safety valve was connected with the pump allowing the subjects to stop the infusion at any time. The system was calibrated before the probe was inserted into the esophagus. Non-linearity of the CSA was corrected for in the whole measurement range by means of a software feature. The pressure was measured by means of a low-compliance perfusion system connected to external transducers.
Sensory ratings
The sensory intensity was assessed continuously during the experiment using an electronic visual analog scale (VAS, GMC, Hornslet, Denmark). The volunteers were trained in assessment of sensation to deep pressure at the muscles on the right forearm several times before the visceral stimuli were given. A scale for both non-painful and painful sensations was used[3]. The intensities of thenon-painful sensations were scored with the following descriptors added to facilitate the scoring: 1 = vague perception of mild sensation; 2 = definite perception of mild sensation; 3 = vague perception of moderate sensation; 4 = definite perception of moderate perception; 5 = the pain threshold. For the painful sensations the patients used the scale from 5 to 10 anchored at 5 = pain threshold to 10 = unbearable pain, with the following anchor words: 6 = slight pain; 7 = moderate pain; 8 = medium pain intensity; 9 = intense pain; and 10 = unbearable pain. This part of the scale was red to clearly separate the non-painful and painful range of sensations. The first three distensions were used to practice the sensory ratings[3]. The subjects were carefully instructed to score the evoked chest pain and to differentiate this from the unpleasantness in the throat caused by traction due to the distension-evoked esophageal contractions. The scale has previously been shown to be robust, and to discriminate sensations in the esophagus[8,11,12], and the small and large intestine[13,14,23,24].
After the last distension before butylscopolamine injection (see below) the volunteers were asked about referred pain to the chest or other remote areas evoked by the distensions at moderate pain (VAS = 7). If present the referred pain area was marked with a pen and transferred to a transparent paper. Later the area was digitized (ACECAD D900+ Digitizer, Taiwan) and the size calculated (Sigma-Scan, Jandel Scientific, Canada).
Protocol
The subjects fasted for at least 4 h prior to the experiment. Intubation was performed through the mouth. The bag was inserted into the stomach and then retracted to identify the location of the lower esophageal sphincter as a zone of high resting pressure that decreased with swallowing. Then the bag was placed 7 cm proximal to the sphincter and the probe was taped to the cheek. The subjects were asked to lie down with the head tilted by 30o after the placement of the bag. The experiment was performed in that position after 30 min of rest.
Three bag distension stimuli with a constant infusion rate of 25 mL/min were done to precondition the tissue and to obtain repeatable sensory data[3,13,15,23,24]. The inter-stimulus interval was 60 s for all experiments. When the subjects reported slight pain (6 on the VAS), the bag was deflated using the same flow rate as during the inflation until it was empty. After these stimuli, two more distensions were done at the same infusion rate. When moderate pain intensity (7 on the VAS) was reached, the pump was reversed and the bag deflated. Then 20 mg butylscopolamine was given intravenously and after abolishment of the contractile activity the two last distensions were repeated.
After the first series of mechanical stimuli, the participants underwent a modified acid perfusion test[25]. Hence, during a perfusion channel in the catheter 0.1 mol/L hydrochloric acid was infused at a rate of 7 mL/min for 30 min. If the evoked sensations due to the acid stimulation were reported unpleasant (rated ≥5 on the VAS), the perfusion was stopped for 30 s and the subjects were allowed to swallow 10 mL water. In case the perfusion was too unpleasant for the subjects, it was stopped and the amount of infused acid was measured.
After the acid perfusion, bag distensions before and after butylscopolamine were given using the same protocol as described above, before acid perfusion.
During all stimuli autonomic reactions were monitored and displayed on-screen using a Biopac MP100 system (Biopac Systems Inc., Santa Barbara, CA, USA) including sensors and recording system for electrocardiogram, pulse rate and respiration.
Data analysis
The circumferential wall tension was calculated according to the law of Laplace for cylindrical structures as
T = △Pr
where T is the circumferential wall tension, r is the balloon radius, and △P is the transmural pressure. The geometry of the esophagus during distension can be considered circular except at very low pressure levels[18]. Therefore, the radius was determined as
Math 1
All subjects stated that they more reliably rated the sensory intensity at the second distension compared to the first. Therefore, only data from the second distension were used in the analysis. After butylscopolamine the first distension was used as the maximal decrease in contractile activity was seen at the first few minutes after the injection.
As criteria for valid contractions before and after acid perfusion a pressure amplitude above 2.5 kPa was used.
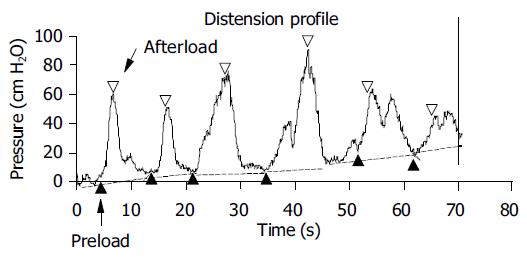
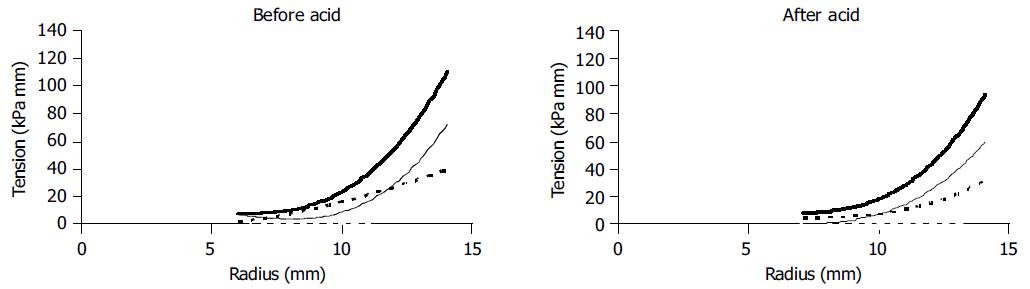
In a representative sample of 10 subjects (5 males and 5 females, mean age 36.1±14.3 years) the change in tension during individual distension-induced contractions (afterload tension) was computed and expressed as function of the radius immediately before the contractions (preload radius). The diagrams were made before and after perfusion of the distal esophagus with a mean of 123 mL hydrochloric acid. An example from an individual subject is shown in Figure 1. The data were fitted with a third order polynomial. These diagrams correspond to the well-known heart ventricular function curves in terms of the ventricular stroke working as function of the mean atrial length. Such curves demonstrate the Frank-Starling mechanism of the heart now adapted to the esophagus-see appendix.
Figure 1 (PDF) Raw data in a typical subject showing the change in pressure during bag-distension-induced contractions.
The change in tension during maximal distension-induced contractions (afterload tension) was computed at the open triangles and expressed as function of the radius immediately before the contractions marked with solid triangles (preload radius). The radius was calculated on the basis of the CSA measured simultaneously. For details regarding the calculations see Methods section.
The pressure and CSA data obtained between the evoked contractions (without infusion of butylscopolamine) were used to compute the total tonic tension, whereas the tracings during butylscopolamine infusion were used for calculation of the passive tension. The active tonic tension was obtained by subtracting the passive tension from the total tonic tension[14,18].
Statistical analysis
The results are expressed as mean±SD unless otherwise indicated. Continuous data were analyzed using t-tests. For multiple comparisons, two-way analysis of variance (ANOVA) was used with the factors: (1) before and after acid and (2) the different VAS levels. P<0.05 was considered significant. The software package SPSS v. 10.0 was used for the statistical analysis.
RESULTS
Mechanical stimuli before and after acid
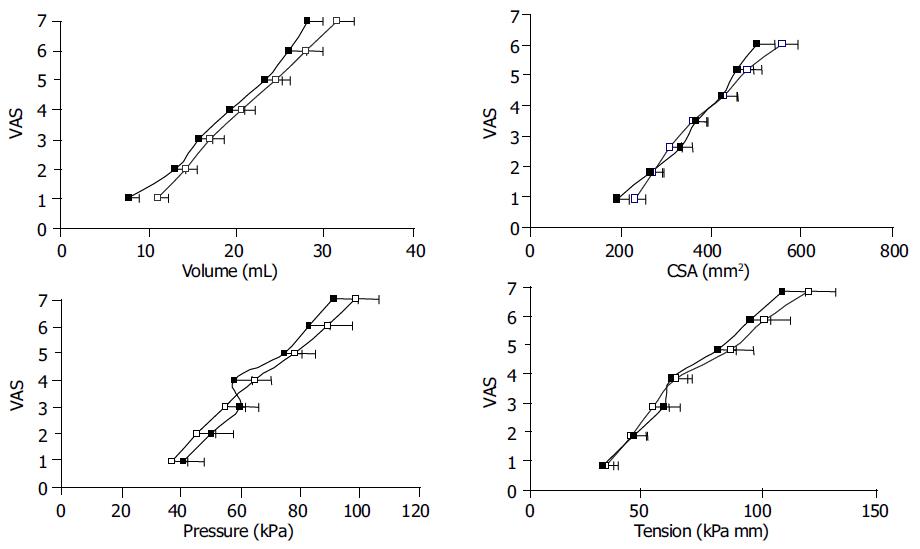
All subjects completed the experiment. After the preconditioning stimuli, the curve characteristics and sensory ratings were reproducible in all subjects. The stimulus-response curves after preconditioning the tissue are shown in Figure 2 for the infused volume, CSA, pressure, and tension. The sensation intensity was approximately linear as functions of all four stimulation variables. The sensory rating increased after acid, when expressed as a function of the volume (F = 4.75, P = 0.03), whereas no differences were found for the CSA (F = 1.0, P = 0.3), pressure (F = 0.7, P = 0.4) and tension (F = 1.2, P = 0.3). The curves during butylscopolamine infusion showed the same pattern as described above, before and after acid perfusion (data not shown).
Figure 2 (PDF) Sensory response to bag distension of the distal esophagus expressed as functions of the volume, CSA, pressure and tension.
The curves were drawn before (□) and after (■) perfusion of the distal esophagus with acid. The sensory response increased after acid perfusion when expressed as a function of the volume.
The acid infusion resulted in a more hyper-reactive esophagus as the number of contractions with pressure amplitudes above 2.5 kPa during the distensions increased from 2.9±1.5 to 3.5±1.5 after acid perfusion (P = 0.03).
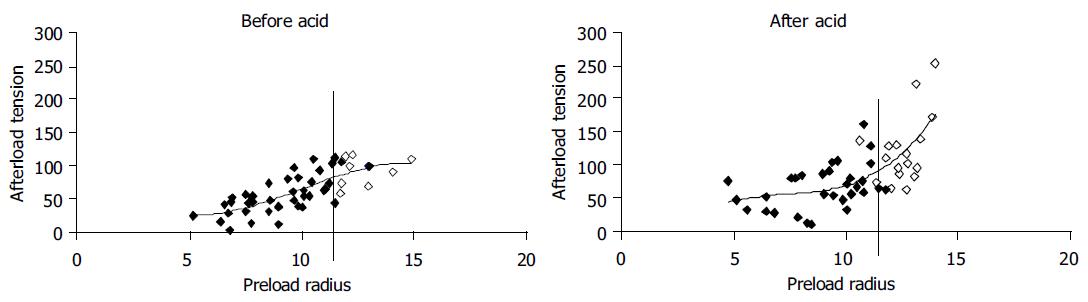
The change in tension during bag-distension-induced contractions (the afterload tension) was plotted as a function of the preload radius for 10 representative subjects (Figure 3). No contractions were observed at radii below 5 mm. Before acid infusion the afterload tension increased until a plateau was reached. This corresponds to the "Frank-Starling mechanism" relating to the less interdigitation of muscle filaments when the muscles are overstretched. Painful sensations (VAS ≥5) were experienced at preload radii higher than 11.5 mm. After the acid infusion higher afterload tensions were observed at both low and high radii as compared to baseline, and there was a tendency to more spreading of the data as some individuals obtained very high afterload tensions. The painful sensations were also only evoked at radii higher than 11.5 mm.
Figure 3 (PDF) Tension during bag-distension-induced contractions (the afterload tension) plotted as functions of the radius immediately before the contractions (the preload radius) as shown in Figure 1.
Five to eight datasets were computed during the distension for 10 representative subjects. Data calculated at painful sensations were all above preload radii of 11.5 mm and are shown as open markings.
The total, passive and active tonic tensions before and after acid infusion are shown in Figure 4. There was no difference in curve shape between before and after acid, indicating that acid infusion does not change esophageal muscle tone.
Figure 4 (PDF) Plots of the total tonic (top), the passive (dotted) and the active (thin) tonic tensions as functions of radius before and after acid infusion.
For explanations see text. Acid did not change the curve shapes. Hence, esophageal muscle tone was not affected by the acid infusion.
High and low acid responders
The subjects tolerated a mean of 101±53 mL of acid. To see if the sensory response was related to the amount of acid infused, the subjects were divided into two groups. One group could accept 100-200 mL of acid (n = 17) and the other group, less than 100 mL of acid (n = 13). There was a relation between the evoked sensitization and the acid load as those who accepted more than 100 mL were sensitized to volume (F = 5.3, P = 0.02), pressure (F = 5.5, P = 0.02) and tension (F = 6.0, P = 0.01), but not to CSA (F = 0.9, P = 0.3). The group tolerating less than 100 mL were not sensitized to neither volume (F = 0.3, P = 0.6), pressure (F= 0.6, P = 0.4), tension (F = 0.8, P = 0.4) nor CSA (F<0.01, P = 0.99).
Referred pain areas
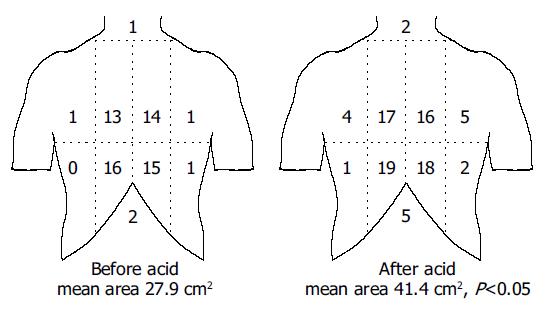
All subjects reported referred pain to the stimulations. The referred pain areas to mechanical stimuli at moderate pain are shown in Figure 5. Additionally one male and two females had referred pain in the back. The referred pain areas increased from 27.9±29.3 cm2 before acid to 41.4±39.0 cm2 after acid (P = 0.047), although the volume was smaller at the distensions after acid.
Figure 5 (PDF) A schematic illustration of the referred pain areas drawn by the subjects following mechanical stimuli of the esophagus before and after perfusion with acid.
The stimuli were given with an intensity corresponding to moderate pain. The chest was divided into eight areas by a horizontal line 5 cm above the xiphoid process, a vertical midline and two vertical lines 5 cm lateral to the midline. The numbers on the figure refer to the number of subjects reporting referred pain to that particular region of the chest. The referred pain area increased following the sensitization with acid.
DISCUSSION
The current experiment used controlled ramp distensions and preconditioning to evoke experimental pain in the esophagus in 30 subjects. The sensory response was assessed before and after sensitization of the lower esophagus by acid perfusion. The sensory rating increased after acid when expressed as a function of the volume, and the degree of sensitization was related to the infused volume of acid. Furthermore, an increase in referred pain to a standardized distension was seen reflecting activation of central facilitatory pain mechanisms. The mechanical analysis demonstrated hyper-reactivity of the esophagus following acid perfusion, with an increased number and force of the phasic contractions, but the muscle tone did not change. This illustrates that acid perfusion not only sensitizes the sensory pathways, but also facilitates motor reflexes.
Sensory response to sensitization with acid
Chronic pain is associated with modifications of the central nervous system such as central sensitization[4]. Animal experiments have demonstrated neuronal changes such as increased spontaneous activity, decreased firing threshold, and expansion of the receptive fields of spinal neurons subjected to activation and/or experimental sensitization of their peripheral afferents[5,26,27]. Sensitization of the human esophagus with acid is a valuable experimental pain model, as the evoked allodynia, hyperalgesia and referred pain patterns reflect sensitization of the nervous system and can be studied systematically[3]. Hence, decreased thresholds to physiologic stimuli seem to contribute to many of the symptoms reported by patients with inflammatory and functional diseases in the gut[28,29]. Thus, a combination of mechanical stimulation and sensitization of the esophagus may mimic the widespread pain and other sensations reported by patients with reflux disease and unexplained chest pain[16,30,31].
Acid-sensitive fibers have been demonstrated in animal studies, and mucosal afferents are often sensitive to different chemical stimuli[32,33]. Increased responses to mechanical, electrical and thermal stimuli after acid perfusion of the esophagus have also been demonstrated in human beings[6-9,34], although previous studies using latex balloons were not consistent. This can be due to methodological problems using latex balloons, where the distension data must be corrected for the intrinsic mechanical properties of the balloons and for the uncontrollable deformation in longitudinal direction[9,16]. Non-compliant polyester urethane bags overcome these problems. The effect of preconditioning the tissue by several distensions until the stress-strain relationship becomes reproducible has also not been considered in most previous studies[3,18]. Different modifications of the acid perfusion test have been used as a chemogenic stimulus by several authors[6,7,9,35,36]. When the current material was divided into those who tolerated below and above 100 mL of acid, significant increased sensation to the mechanical stimulus was only seen in the high acid group. Hence, it is recommended to use volumes higher than 100 mL in future studies.
The present study demonstrated increased sensation to the infused bag volume, but not to pressure and tension. The intraluminal pressure and tension are highly dependent of the contractile force state of the esophageal muscles, and hence not as reliable parameters as the deformation[18]. Despite the decrease in volume after acid infusion, the CSA did not decrease significantly. Thus it seems that the bag conforms to a shape where it is shorter after acid infusion. Such a shape change is likely caused by changes in the contractile activity in the acid exposed area and even in regions affected by nerve-mediated reflex responses.
Secondary contractions and muscle hyper-responsiveness can be evoked by acid in the distal esophagus due to reflex loops between mucosal afferents and the motor system[37-40]. After acid perfusion increased force of the secondary contractions was evoked by the distension in the non-painful and painful range. Animal studies have shown that-in contrast to the somatic system-afferents encoding both non-painful and painful sensations can sensitize in the viscera[41]. The current observations in the human esophagus are in line with these studies, as the sensitization of afferents encoding conscious sensations to distension seems to change the contractile activity in the muscle via local and/or central reflexes[42,43]. However, the curve form changed mostly in the pain range (to the right of the vertical line in Figure 3) and hence there seems to be a higher effect of sensitization on the painful sensations. The Frank-Starling mechanism predicts a decrease in contractile activity when the muscle is overstretched corresponding to less optimal interdigitation of actin and myosin filaments. In the current experiment the baseline curve form showed no decrease in afterload tension at maximal distension. The fact that the afterload tensions were higher after acid infusion indicates that bag distension itself does not activate the muscle maximally.
Another manifestation of the acid perfusion was the increased referred pain area to the mechanical stimulation, although the bag volume was lower after the perfusion. Enlarged referred pain areas is also a characteristic in clinical gut disorders[44-46], and are very similar to what is observed in patients suffering from chronic musculoskeletal pain[47]. Previously, we have shown that sensitization of the esophagus results in increased referred pain areas[8], a finding which was confirmed in the current study. The mechanism is of central origin[4], which was also shown in previous papers using neurophysiological assessment of the spinal and supraspinal pain response after acid perfusion of the esophagus[8,48].
Mechanical and motor responses to sensitization with acid
The sensitization resulted not only in allodynia and hyperalgesia to the distension volumes, but the esophagus also exhibited hyper-reactivity as illustrated by the increased number of contractions after the acid perfusion. Such hyper-reactivity has also been seen in animal studies[37,38]. The contractions were also stronger to a given preload radius. However, the acid infusion did not change the total tonic tension, the passive tension and the active tonic tensions (Figure 4). Hence, the hyper-reactivity only accounts for phasic contractions, not for tone in the esophageal body. Previously, Sifrim et al[49] showed that acid reflux into the esophagus stimulated tone in the esophageal body. However, simultaneous distension seemed to inhibit the acid induced tone. These issues obviously need further investigations.
The preload radius where contractions were evoked by the painful stimuli (VAS = 5 and higher) did not change after acid (vertical line in Figure 2). This corresponds with the "strain theory", i.e., that the mechanoreceptors are activated by circumferential stretch independent of the contractile state of the muscles[12,13,15,50]. The receptors encoding distension of the gut are mainly believed to be localized in the muscle and nerve layers, where they are not exposed to acid[33,43]. Hence, the contractions are probably initiated by reflex loops between strain-sensitive mechanosensitive afferents localized in the muscle layers and the smooth muscle cells. Whether such reflexes are local or mediated via central (vagal and/or spinal) afferents cannot be concluded from the current data[40,42,43]. We believe, however, that a central component is important as the contractions were more powerful after perfusion with acid. The acid perfusion may thus result in sensitization of mucosal afferents as well as central hyperexcitability[8,43,48]. As the enteric nervous system is partly under inhibitory central control[51], the sensitization may result in dampening of the central control. This mechanism is expedient as such reflexes will tend to move acid from reflux towards the stomach where it is harmless.
Modeling diseases of the esophagus
Sensation and pain detection thresholds to distension, electrical and acid stimuli of the esophagus were found to be lower in patients with non-cardiac chest pain compared to healthy subjects[6,7,52,53]. Such hypersensitivity can be mimicked in the current model. Furthermore, the muscles of the esophagus are hyper-reactive in patients with unexplained chest pain[19,54-56]. In the present model the acid perfusion evoked an increased number of contractions, which were characterized by a higher force. Thus diseases characterized by primary and secondary motor disorders can also be mimicked experimentally, and in patients the preload-afterload plots will be valuable for description of the aberrant motor function. The model can therefore be used to study abnormal sensory-motor mechanisms in visceral organs, and may also prove useful in pharmacological studies with drugs targeted to treat patients with unexplained chest pain and motor disorders of the esophagus.
Appendix
The preload is considered in this study to be initial muscle length (radius) preceding the contraction during the distension, whereas the afterload is evaluated as the active tension during the contraction. In cardiac physiology the preload is usually considered to be the end-diastolic pressure or radius and the afterload is considered to be the arterial pressure during the systole. The explanation of the Frank-Starling mechanism is that when an extra amount of blood flows into the ventricles, the cardiac muscle itself is stretched to greater length. This in turn causes the muscle to contract with increased force because the actin and myosin filaments then are brought to a more nearly optimal degree of interdigitation for force generation. In cardiac physiology the importance of the concept of preload and afterload is that in many abnormal functional states of the heart and circulation, the pressure during filling of the ventricle or the arterial pressure against which the ventricle must contract, or both, are severely altered from the normal. The Frank-Starling mechanism has been important in the understanding of drugs with effect on the myocardial function, and transferring this concept to esophageal physiology, the development in the current model will have interest for evaluation of normal esophageal physiology and in the pathophysiology of esophageal disorders.