Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3948
Revised: September 14, 2004
Accepted: September 19, 2004
Published online: July 7, 2005
AIM: To examine the prevalence and prognostic significance of C-kit gene mutation and analysis the correlation of C-kit gene mutation and the clinicalpathologic parameters of GISTs.
METHODS: Eighty-two GISTs were studied for the mutation of C-kit gene by PCR-SSCP, DNA sequence. Statistical comparison were used to analysis the correlation of C-kit gene mutation and clinicalpathology, clinical behavior, recurrence.
RESULTS: (1) Mutation-positive and mutation-negative GISTs were 34 and 48,respectively; (2) Among these patients with C-kit mutation remained a significantly poor prognosis associated with 59% 3-year survival compared to those whose tumors did not; (3) Tumor size, PCNA index, mitotic cell number, presence of necrosis, microscopic invasion to adjacent tissues, recurrence and distant metastasis among mutation-positive and mutation-negative GISTs were significantly different.
CONCLUSION: C-kit mutation is a undoubtedly pivotal event in GIST and may be associated with poor prognosis. Evaluation of C-kit gene mutation may have both prognosis and therapeutic significances.
- Citation: Liu XH, Bai CG, Xie Q, Feng F, Xu ZY, Ma DL. Prognostic value of KIT mutation in gastrointestinal stromal tumors. World J Gastroenterol 2005; 11(25): 3948-3952
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3948.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3948
Gastrointestinal stromal tumor (GIST) is designation for a major subset of mesenchymal tumors of the gastrointestinal tract. Their biological behavior is a persistent source of controversy. Concerning prognosis ,various studies[1-3] have endeavored in the establishment of clinicopathologic correlations, such as tumor size, location, mitotic cell count, proliferative activity, presence of necrosis, presence of hemorrhage, microscopic invasion to adjacent tissues, recurrence, distant metastasis, age, sex, cell type. Yet the criteria claimed to predict the biological behavior of GIST remains vague and do not even enable a confident discrimination between benign and malignant lesions. Discrimination of malignant GISTs based on an objectively defined factor would be of practical importance. In this study, we intend to contribute to this issue by examining the prevalence and prognostic significance of the above-mentioned parameters, C-kit expression and C-kit gene mutation, and analysis the correlation of C-kit gene mutation and the above-mentioned clinical parameters.
During the period 1997-2001, 82 patients with primary mesenchymal tumors of the gastrointestinal tract underwent surgery at Changhai Hospital and were diagnosed as GISTs. Of these, 56 men and 26 women, the mean age of the GIST patients at the time of diagnosis was 53 years. The locations of the tumors were as follows: 3 in the esophagus, 42 in the stomach, 35 in the intestine, and 2 in the mesentery. According to the standard of Lewin’s[4], 82 cases were divided into 2 groups, 20 benign and 62 malignant GISTs. At the time of diagnosis, 16 were found microscopic invasion to mucous myometrium or placenta percreta, distant metastasis was found in 16 patients, 9 to the liver, 2 to bone, 1 to lung and 4 extensive metastasis; the remaining patients were free of distant metastasis. Thus ,curative surgery was performed for 73 patients, and the patients with distant metastasis or peritoneal dissemination underwent only local resection. The mean follow-up period was 4.1 years, during the follow-up period, 18 patients suffered from recurrences, and 10 patients received reoperation for recurrences.
Paraffin sections (3 mm thick) of formalin-fixed tissues were used for conventional H&E staining and for immunohistochemistry. Rabbit polyclonal antibody against human KIT and mouse monoclonal antibody against human PCNA were purchased from DAKO. Immunohistochemistry was performed using EnVision kit by two-step technique. PCNA index indicating the percentage of positive tumor nuclei was obtained by visual quantitative assessment of 100 tumor cells. Random fields were selected, excluding areas with excessive or scant immunostaining. Nuclear staining without cytoplasmic staining was considered a positive result. Negative result was set as “-”, PCNA index < 10% +, PCNA index 10%-30%++, > 30% +++.
DNA was extracted from formalin-fixed, paraffin-embedded tissues using standard methods with proteinase K digested and phenol/chloroform purified. Exon11 of the C-kit gene was amplified by PCR using the following oligonucleotide primer pairs: sense primer 5’-aactcagcctgtttctgg-3’-antisense primer: 5’-gatctatttttccctttctc-3’. PCR products were subjected to 8% non-denaturation polyacrylamide gel electrophoresis (aer:bis = 49:1) with 5% glycerin and silver nitrate staining.
PCR products that showed abnormal gel shift by PCR-SSCP were selected for sequencing after cloned into PMD18-T vector. The sequencing procedures were performed by Songon Co., Shanghai.
Eighty-two GISTs were divided into mutation-positive and mutation-negative subtypes according to C-kit gene detection. χ2 test was used to analyze the correlation of C-kit mutation and clinicopathological and prognostic factors. Two-sided P < 0.05 were considered to represent statistical significance. The relative importance of various prognostic factors for the postoperative was analyzed with Cox’s proportional hazard model. Logistic regression analysis with the forward stepwise method was used to compare the difference of survival curves between the C-kit mutation-positive and mutation-negative subtypes. The possible prognostic factors included age, sex, location of tumors, tumor size, cell type, microscopic invasion to neighboring structures, mitotic cell number, PCNA index, distant metastasis at the time of diagnosis, peritoneal dissemination at the time of surgery, recurrences during the follow-up period, presence of necrosis, presence of hemorrhage, presence of C-kit mutation and expression.
Immunohistochemical analyses revealed strong and diffuse C-kit expression in 80 of 82 cases. PCNA staining intensity varied from very weak to intensely strong and from finely granular to uniformly dark red-brown (Table 1).
| Clinicalpathological | Total | C-kit | C-kit | χ2 | P | ||
| Case | Percent | Case | Percent | ||||
| Total | 82 | 34 | 48 | ||||
| Age (yr) | |||||||
| <40 | 12 | 5 | 15 | 7 | 15% | 0.0002 | 0.9877 |
| 40 | 70 | 29 | 85 | 41 | 85% | ||
| Sex | |||||||
| Man | 56 | 24 | 71 | 32 | 67% | 0.1413 | 0.7069 |
| Woman | 26 | 10 | 29 | 16 | 33% | ||
| Tumor size | |||||||
| 1-5.4 | 46 | 10 | 29 | 36 | 75% | 16.795 | < 0.0001 |
| 5.5 | 36 | 24 | 71 | 12 | 25% | ||
| Location of tumors | |||||||
| Esophagus | 3 | 0 | 0 | 3 | 6% | ||
| Stomach | 42 | 14 | 41 | 28 | 58% | 7.5243 | 0.0569 |
| Intestine | 35 | 18 | 53 | 17 | 36% | ||
| Mesentery | 2 | 2 | 6 | 0 | 0% | ||
| Cell type | |||||||
| Spindle | 73 | 29 | 85 | 44 | 92% | 0.8272 | 0.3631 |
| Epithelioid | 9 | 5 | 15 | 4 | 8% | ||
| PCNA index | |||||||
| 0 | 19 | 7 | 21 | 12 | 25% | ||
| + | 49 | 15 | 44 | 34 | 71% | 17.099 | 0.0007 |
| ++ | 13 | 12 | 35 | 1 | 2% | ||
| +++ | 1 | 0 | 0 | 1 | 2% | ||
| Mitotic | |||||||
| <5 | 67 | 22 | 65 | 45 | 94% | 11.233 | 0.00008 |
| 5/50 | 15 | 12 | 35 | 3 | 6% | ||
| Hemorrhage | |||||||
| Yes | 25 | 12 | 35 | 13 | 27% | 0.6331 | 0.4262 |
| No | 57 | 22 | 65 | 35 | 73% | ||
| Necrosis | |||||||
| Yes | 37 | 20 | 59 | 17 | 35 | 4.4036 | 0.0359 |
| No | 45 | 14 | 41 | 31 | 65 | ||
| Microscopic | |||||||
| Yes | 16 | 15 | 44 | 1 | 2 | 22.391 | < 0.0001 |
| No | 66 | 19 | 56 | 47 | 98 | ||
| C-kit expression | |||||||
| Positive | 80 | 33 | 97 | 47 | 98 | 0 | 1 |
| Negative | 2 | 1 | 3 | 1 | 2 | ||
Two alleles were observed for the normal PCR segments, fast and slow fragments were designated as A and B alleles, respectively. Comparing to normal cases, 34 (41.5%) malignant GISTs showed abnormal gel shifts while no mutant bands were observed in benign GISTs. Abnormal gel shifts included multiple shift bands, monomorphic fragment or band shifting. Sequencing of abnormal bands revealed point mutation, deletion mutation and insertion deletion. Mutation-positive and mutation-negative GISTs were 34 and 48, respectively.
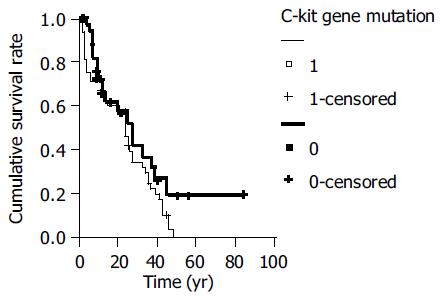
Three-year survival rate was 77%(63/82) of 82 cases. Among these patients with C-kit mutation remained a significantly poor prognosis associated with 59% 3-year survival compared to those whose tumors did not. (χ2 = 11.6464, P < 0.05, Figure 1).
We examined whether the presence of C-kit mutation could be associated with clinicopathological features of GISTs (Table 1).Statistical analysis indicated that significant differences were detected with tumor size (χ2 = 16.7950, P < 0.05), PCNA index (χ2 = 17.0990, P < 0.05), mitotic cell number (χ2 = 11.2327, P < 0.05), presence of necrosis (χ2 = 4.4036, P < 0.05), microscopic invasion to adjacent tissues (χ2 = 22.3912, P < 0.05) between two terms. The age, sex, location of tumor, cell type, the presence of hemorrhage, and C-kit expression were independently related to the presence of C-kit mutation.
We also examined whether the presence of C-kit mutation was associated with the clinical outcome (Table 2). The mutation-positive GISTs showed more frequent recurrences (χ2 = 16.333, P < 0.05), distant metastasis (χ2 = 12.9649, P < 0.05) and higher mortality (χ2 = 11.6464, P < 0.05) than the mutation-negative GISTs. Of the patients with mutation-positive GIST ,developed distant metastasis.
| Prognostic | Total | C-kit mutation-positive subtype | C-kit mutation-negative subtype | χ2 | P | ||
| Case | Percent | Case | Percent | ||||
| Total | 82 | 34 | 48 | ||||
| Recurrence | |||||||
| Yes | 18 | 13 | 38 | 5 | 10 | 16.333 | 0.0001 |
| No | 64 | 21 | 62 | 43 | 90 | ||
| Metastasis | 16 | 13 | 38 | 3 | 6 | 12.9649 | 0.0003 |
| Live | 10 | 9 | 26 | 1 | 2 | ||
| Abdominal cavity | 3 | 2 | 6 | 1 | 2 | ||
| Distant | 3 | 2 | 6 | 1 | 2 | ||
| Prognosis | |||||||
| Survival | 63 | 20 | 59 | 43 | 90 | 11.6464 | 0.0006 |
| Death | 19 | 14 | 41 | 5 | 10 | ||
GISTs are the most common mesenchymal tumors of the human gastrointestinal tract which are enigmatic in terms of their line of differentiation or cell of origin and clinical behavior[5]. Recently, many studies have shown that tumor size, location, mitotic cell count, cell type, proliferative activity, presence of necrosis, presence of hemorrhage, microscopic invasion to adjacent tissues, recurrence, distant metastasis, as favorable factors of GIST. But histologic distinction of benign from malignant tumors is often difficult, and prediction of clinical outcome on the basis of histologic characteristics alone is not always reliable, many histologically malignant neoplasms were clinically benign. Moreover, some tumors had conflicting features of both benignancy and malignancy, and their malignant potential has been labeled uncertain or indeterminate. In recent years, C-kit protein expression and activated gene mutation have been found in GIST[6-9], aggressive mastocytosis[10], mast cell leukemia[11], ANLL with/without mast cell involvement [10], myeloproliferative disorders[10], colon carcinoma[12] and germ cell tumors[13]. Dections of gene mutation have shown a good correlation with biologic behavior in such tumors and providing valuable adjunctive prognostic information. In this study, we examined whether the presence of mutation of C-kit gene is important as a prognostic factor of GIST and found it to indicate a significantly poorer prognosis.
First, we examined whether the presence of C-kit mutation could be associated with clinicopathological features of GISTs. Statistical analysis indicated that significant differences were detected with tumor size, PCNA index, mitotic cell number, presence of necrosis, microscopic invasion to adjacent tissues between mutation-positive and mutation-negative GISTs. While the age, sex, location of tumor, cell type, the presence of hemorrhage, and C-kit expression were independently related to the presence of C-kit mutation. This states that the mutation-positive GISTs were larger in size and showed more frequent invasion of adjacent tissues, the mutation-positive GISTs showed higher mitotic counts and PCNA index and more necrosis within the tumors histologically. Taken together, the mutation-positive GISTs had more malignant histological features than the mutation-negative GISTs. We also examined whether the presence of C-kit mutation was associated with the clinical outcome. The mutation-positive GISTs showed more frequent recurrences, distant metastasis and, higher mortality than the mutation-negative GISTs. There are at least possible two explanations for this: GIST may be classified into two subtypes, C-kit mutation-positive and mutation-negative GISTs mutation-positive GISTs showed more malignant features,; mutation-negative GISTs may acquire more malignant phenotype due to the addition of C-kit mutation.
Recently, gain-of-function mutations of the juxtramemebrane domain of C-kit , including deletions or point mutations in exon11 were described in GIST[14-18]. These mutations were shown to lead to spontaneous, lilgand-independent tyrosine kinase activation. The stable transfection of murine lymphoid cells with the mutant C-kit complementary DNA was shown to induce malignant transformation, suggesting pathogenetic significance of such mutations. We have expanded these observations and found that the C-kit mutation in the exon11 predominantly occur in those GISTs that are histologically and clinically malignant while benign GISTs were not detected. This suggest that C-kit mutation in the exon11 may be an important pathogenetic mechanism of tumor malignant behavior. Moreover ,we found C-kit mutations only in 60% of the malignant GISTs. This may suggest that additional pathogenetic mechanisms exist in other cases, or that mutations occur in alternative sites. Another study also showed correlation between the mutations and disease progression.
There is no effective therapy for unresectable or metastatic gastrointestinal stromal tumor, which is invariably fatal. STI571, a phenylaminopyrimidine derivative, is a small molecule that selectively inhibits the enzymatic activity of C-kit gene. Joensuu et al[19] found that inhibition by STI571 of the constitutively active mutant C-kit tyrosine kinase of gastrointestinal stromal tumors was an effective therapy for these tumors. There are two explanations for this: STI571 may be active in solid tumors that rely on the expression of C-kit, ABL, or platelet-derived growth factor receptor while GIST uniformly express C-kit; a tumor-specific C-kit mutation appears to be the chief cause of this neoplasm. Considering these findings, we conclude that C-kit mutation is a undoubtedly pivotal event in GIST and may be associated with poor prognosis. Evaluation of C-kit gene mutation may have both prognosis and therapeutic significances as the new tyrosine kinase inhibitor (STI571) treatments are available.
Co-first-authors: Qiang Xie Co-correspondents: Zhi-Yun Xu
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Wong NA, Young R, Malcomson RD, Nayar AG, Jamieson LA, Save VE, Carey FA, Brewster DH, Han C, Al-Nafussi A. Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology. 2003;43:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Nagasako Y, Misawa K, Kohashi S, Hasegawa K, Okawa Y, Sano H, Takada A, Sato H. Evaluation of malignancy using Ki-67 labeling index for gastric stromal tumor. Gastric Cancer. 2003;6:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Kim MK, Lee JK, Park ET, Lee SH, Seol SY, Chung JM, Kang MS, Yoon HK. [Gastrointestinal stromal tumors: clinical, pathologic features and effectiveness of new diagnostic criteria]. Korean J Gastroenterol. 2004;43:341-348. [PubMed] |
| 4. | Lewin KJ, Riddle R, Weinstein W. Gastrointestinal Pathology and Its Clinical Implications. 2thnd. New York: Igaku-Shoin Medical Pub 1992; 284-341. |
| 5. | Ballarini C, Intra M, Ceretti AP, Prestipino F, Bianchi FM, Sparacio F, Berti E, Perrone S, Silva F. Gastrointestinal stromal tumors: a "benign" tumor with hepatic metastasis after 11 years. Tumori. 1998;84:78-81. [PubMed] |
| 6. | Goldblum JR. Gastrointestinal stromal tumors. A review of characteristics morphologic, immunohistochemical, and molecular genetic features. Am J Clin Pathol. 2002;117 Suppl:S49-S61. [PubMed] |
| 7. | Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 838] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 8. | Ma DL, Liu XH, Bai CG, Xie Q, Feng F. Effect of c-kit gene mutation on prognosis of gastrointestinal stromal tumor. Zhonghua Waike Zazhi. 2004;42:140-144. [PubMed] |
| 9. | Théou N, Tabone S, Saffroy R, Le Cesne A, Julié C, Cortez A, Lavergne-Slove A, Debuire B, Lemoine A, Emile JF. High expression of both mutant and wild-type alleles of c-kit in gastrointestinal stromal tumors. Biochim Biophys Acta. 2004;1688:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Pardanani A, Reeder TL, Kimlinger TK, Baek JY, Li CY, Butterfield JH, Tefferi A. Flt-3 and c-kit mutation studies in a spectrum of chronic myeloid disorders including systemic mast cell disease. Leuk Res. 2003;27:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Noack F, Escribano L, Sotlar K, Nunez R, Schuetze K, Valent P, Horny HP. Evolution of urticaria pigmentosa into indolent systemic mastocytosis: abnormal immunophenotype of mast cells without evidence of c-kit mutation ASP-816-VAL. Leuk Lymphoma. 2003;44:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Sammarco I, Capurso G, Coppola L, Bonifazi AP, Cassetta S, Delle Fave G, Carrara A, Grassi GB, Rossi P, Sette C. Expression of the proto-oncogene c-KIT in normal and tumor tissues from colorectal carcinoma patients. Int J Colorectal Dis. 2004;19:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Looijenga LH, de Leeuw H, van Oorschot M, van Gurp RJ, Stoop H, Gillis AJ, de Gouveia Brazao CA, Weber RF, Kirkels WJ, van Dijk T. Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ-cell tumors. Cancer Res. 2003;63:7674-7678. [PubMed] |
| 14. | Beghini A, Tibiletti MG, Roversi G, Chiaravalli AM, Serio G, Capella C, Larizza L. Germline mutation in the juxtamembrane domain of the kit gene in a family with gastrointestinal stromal tumors and urticaria pigmentosa. Cancer. 2001;92:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Lee JR, Joshi V, Griffin JW, Lasota J, Miettinen M. Gastrointestinal autonomic nerve tumor: immunohistochemical and molecular identity with gastrointestinal stromal tumor. Am J Surg Pathol. 2001;25:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Fukuda R, Hamamoto N, Uchida Y, Furuta K, Katsube T, Kazumori H, Ishihara S, Amano K, Adachi K, Watanabe M. Gastrointestinal stromal tumor with a novel mutation of KIT proto-oncogene. Intern Med. 2001;40:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297-4300. [PubMed] |
| 19. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1326] [Article Influence: 55.3] [Reference Citation Analysis (0)] |