Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3882
Revised: December 15, 2004
Accepted: December 20, 2004
Published online: July 7, 2005
AIM: To examine the correlation between the porto-systemic hypertension evaluated by portal shunt index (PSI) and life-threatening complications, including hepatocellular carcinoma (HCC), liver failure (Child-Pugh stage progression), and esophagogastric varices.
METHODS: Two hundred and twelve consecutive subjects with HCV-related cirrhosis (LC-C) underwent per-rectal portal scintigraphy. They were allocated into three groups according to their PSI: group I, PSI ≤ 10%; group II, 10%<PSI<30%; and group III, 30% ≤ PSI. Of these, selected 122 Child-Pugh stage A (Child A) subjects were included in analysis (a mean follow-up period of 5.9 ± 5.4 years, range 6 mo-21 years).
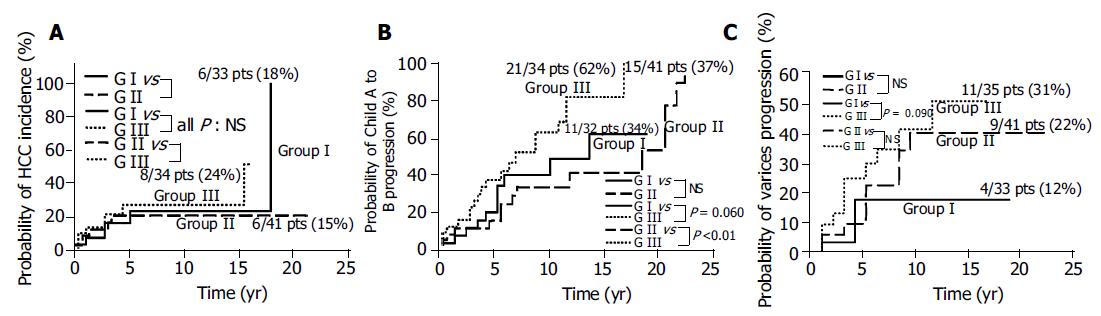
RESULTS: No significant correlation between PSI and cumulative probability of HCC incidence was observed. Cumulative probability of Child A to B progression was tended to be higher in group III than in group I, and significantly higher in group III than in group II (62% vs 34%, 62% vs 37%; P = 0.060, <0.01; respectively). Cumulative probability of varices tended to be higher in group III than in group I (31% vs 12%, P = 0.090). On multivariate analyses, significant correlation between PSI and Child A to B progression was observed, and no significant correlation between PSI and HCC incidence or varices progression was observed.
CONCLUSION: Patients with LC-C of Child A will progress to Child B rapidly after their PSI reaches 30% or higher. PSI can be used to predict occult progressive porto-systemic shunting and liver failure non-invasively. It indicates that PSI may play an important role in follow-up of the porto-systemic hypertension gradient for outpatients with LC unlike hepatic venous catheterization.
- Citation: Kawamura E, Habu D, Hayashi T, Oe A, Kotani J, Ishizu H, Torii K, Kawabe J, Fukushima W, Tanaka T, Nishiguchi S, Shiomi S. Natural history of major complications in hepatitis C virus-related cirrhosis evaluated by per-rectal portal scintigraphy. World J Gastroenterol 2005; 11(25): 3882-3886
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3882.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3882
Hepatitis C virus (HCV) is the most common cause of chronic liver disease in several countries, including Japan, and chronic hepatitis due to HCV (CH-C), which exhibits a variable natural course, is becoming a subject of worldwide interest. CH-C progresses to cirrhosis of the liver (LC), and may be complicated by hepatocellular carcinoma (HCC), hepatic decompensation, and esophagogastric varices[1,2], although its clinical course has not been fully defined. Despite treatment such as injection of interferon plus oral ribavirin[3], many patients with CH-C progress to cirrhosis[4], and develop portal hypertension as CH-C advances to the early phase of LC[5].
Portal hypertension evaluated by “invasive” hepatic venous pressure gradient (HVPG) is associated with progression of liver failure and death[6-8]. Using the method described in this study, the extent of porto-systemic shunting (PSS) can be evaluated with the portal shunt index (PSI) using relatively “non-invasive” per-rectal portal scintigraphy with 99mTc pertechnetate, because PSI correlates strongly with portal pressure[9,10]. This study monitored three life-threatening complications of LC, including the incidence of HCC, Child-Pugh stage progression, and progression of esophagogastric varices, and examined the correlation between PSI and these three complications.
A retrospective cohort study was performed on 212 subjects with HCV-related cirrhosis (LC-C), who were admitted to our hospital during the 24 years between March 1979 and June 2002, and who were evaluated with PSI obtained by per-rectal portal scintigraphy with 99mTc pertechnetate. These subjects were diagnosed by examination of liver specimens obtained by laparoscopy, or needle biopsy performed under ultrasonic guidance. Exclusion criteria for this study were as follows: other causes of cirrhosis such as HBV, autoimmune disease, any alcohol consumption; past treatment with interferon, endoscopic sclerotherapy or open surgery for varices; and trans-arterial embolization or open surgery for HCC. Within a week of hospitalization, all subjects underwent abdominal ultrasonography for detection of ascites, Child-Pugh staging as an index of liver failure and endoscopy for detection of esophagogastric varices[11]. Three Child-Pugh stages were considered: stage A (score 5-6), stage B (7-9), and stage C (10-15). The 212 subjects with cirrhosis were distributed as follows: Child-Pugh stage A (Child A), 122; Child B, 73; and Child C, 17. At entry, we used other possible predictors of LC prognosis, including sex, age, serum albumin, total bilirubin (T-bil), prothrombin time (PT), and platelets[12].
We selected 122 Child A subjects for a longitudinal study; these subjects gave their informed consent to participate, and agreed to return after discharge to our outpatient clinic for monitoring. The procedures were approved by the Ethics Committee of Graduate School of Medicine, Osaka City University. A total of 122 subjects were monitored for a mean period of 5.9 ± 5.4 years (range 6 mo to 21 years). Monitoring was maintained for each evaluation until confirmation of HCC, or Child A to B progression, or varices progression, or the end of the outcome observation period (June 2002). Subjects were excluded from the study if they were followed by another hospital, or their monitoring periods were less than 6 mo.
After excluding dropouts, we were able to monitor the following subjects for at least 6 mo: for HCC incidence, 108 subjects; for Child A to B progression, 107; and for varices progression, 109. A PSI value of 10% or higher is considered to be abnormal[9], and a PSI of 30% or higher has an especially poor prognosis for chronic liver diseases[5]. We defined three groups according to their PSI: group I, PSI ≤ 10%; group II, 10% < PSI < 30%; and group III, 30% ≤ PSI. The subjects were further divided as follows: for HCC incidence, 108 subjects-group I, 33; group II, 41; and group III, 34; for Child A to B progression, 107 subjects-group I, 32; group II, 41; and group III, 34; for varices progression, 109 subjects-group I, 33; group II, 41; and group III, 35. These subjects underwent the following examinations: laboratory studies and physical assessment of the extent of hepatic encephalopathy for Child’s staging, with a mean interval of 4.1 ± 0.8 mo; abdominal ultrason-ography or dynamic CT for assessment of the extent of ascites or the existence of HCC, with a mean interval of 2.1 ± 0.6 mo; and endoscopy for varices, with a mean interval of 8.1 ± 2.1 mo. HCC was confirmed by histology obtained by needle biopsy performed under ultrasonic guidance, or confirmed by selective angiography. The extent of hepatic encephalopathy was defined from detection of tremor and/or disorientation by physicians. The extent of ascites was confirmed by abdominal ultrasonography and/or physical assessment. We defined progression (or incidence) of each complication as the first confirmation of HCC, or Child B or a new variceal factor[13]. Figure 1 shows flow of participants through monitoring.
The subjects fasted after the evening meal on the day before examination. In the morning, the rectum was emptied by administration of a laxative. First, 370 MBq of 99mTc pertechnetate (2 mL solution) was given per rectum through a polyethylene tube (Nélaton’s catheter, French 16) into the upper rectum, followed by 15 mL of air. Time–activity curves for the heart and liver areas were obtained every 4 s using a large-field scintillation camera (Vertex-Plus, ADAC Laboratories, Silicon Valley, USA). It was equipped with a low-energy, multipurpose, parallel-hole collimator and was interfaced with a digital computer. The camera was positioned over the patient’s abdomen so that the field of view included the heart, liver, and spleen. At the end of the 5-min examination, a 5-min summed color image was recorded. To measure the extent of PSS by PSI, we calculated the number of counts for the heart as a percentage of the counts for the heart and liver integrated for 24 s immediately after the appearance of the liver time-activity curve[9].
Results were analyzed by SAS 8.12 statistical software (SAS Institute Inc., Cary, NC)[14,15]. Data were expressed as mean±SD. Comparisons between PSI groups were made by the Kruskal-Wallis test, the Mantel-Haenszel test, or ANOVA. The cumulative progression rates were calculated and plotted by the Kaplan-Meier method, and were compared by the log-rank test. Any significant variable was considered suitable for the multivariate analysis using Cox’s regression model. P < 0.05 was taken as statistically significant.
Table 1 presents patient data at entry classified by PSI. The differences between the PSI groups were significant for the following parameters: age, albumin, T-bil, platelets (P < 0.01, < 0.01, < 0.05, and < 0.01, respectively).
| Group I | Group II | Group III | P | ||
| PSI ≤ 10% | 10% < PSI < 30% | 30% ≤ PSI | |||
| Sex (Male/Female), n | 25/13 | 31/15 | 30/8 | NS | 2 |
| Age, yr | 48.4 ± 11.7 | 54.3 ± 13.0 | 54.9 ± 9.9 | < 0.01 | 1 |
| Albumin, g/dL | 4.0 ± 0.5 | 4.0 ± 0.3 | 3.6 ± 0.4 | < 0.01 | 3 |
| Total bilirubin, mg/dL | 0.8 ± 0.3 | 0.9 ± 0.4 | 1.1 ± 0.4 | < 0.05 | 1 |
| Prothrombin time, % | 95.4 ± 17.2 | 94.5 ± 15.5 | 92.5 ± 20.2 | NS | 1 |
| Platelets, /mm3 | 16.4 ± 5.7 | 14.7 ± 6.9 | 10.6 ± 5.0 | < 0.01 | 1 |
No significant correlation between PSI and cumulative probability of HCC incidence was observed (Figure 2A). Cumulative probability of Child A to B progression was tended to be higher in group III than in group I, and significantly higher in group III than in group II (62% vs 34%, 62% vs 37%; P = 0.060, < 0.01; respectively) (Figure 2B). Cumulative probability of esophagogastric varices tended to be higher in group III than in group I (31% vs 12%, P = 0.090) (Figure 2C).
Table 2 presents the proportions of Child A to B progression and relative risks as uni- and multivariates of possible predictors, which were classified at the entry of the study. The total proportion of each predictor, except PSI, was divided into two between better (upper line) and worse (lower line) at a cut-off value according to Child staging, or reports by other authors: for instance, albumin, at 3.5 g/dL[11,12,16].
| Classification of | Proportion of Child-Pugh | 1Relative risks | ||
| predictor at entry | stage A to B progression, n/N (%) | Crude RR (95%CI) | 2Adjusted RR (95%CI) | |
| Sex | Female | 16/34 (47.1) | 1.00 | |
| Male | 31/73 (42.5) | 1.47 (0.76-2.84) | ||
| Age (per 1 yr) | 0.99 (0.96-1.01) | |||
| Albumin, g/dL | 3.5+ | 25/63 (39.7) | 1.00 | 1.00 |
| < 3.5 | 22/44 (50.0) | 1.49 (0.83-2.67) | 0.98 (0.49-1.95) | |
| Total bilirubin, mg/dL | < 1.0 | 32/69 (46.4) | 1.00 | |
| 1.0+ | 15/38 (39.5) | 1.27 (0.68-2.39) | ||
| Prothrombin time, % | 100+ | 27/66 (40.9) | 1.00 | |
| < 100 | 20/41 (48.8) | 1.06 (0.59-1.91) | ||
| Platelets, /mm3 | 10+ | 32/73 (43.8) | 1.00 | 1.00 |
| < 10 | 13/32 (40.6) | 1.6 (0.81-3.16) | 1.40 (0.68-2.86) | |
| Portal shunt index | Group I | 11/32 (34.4) | 1.51 (0.65-3.51) | 1.67 (0.70-3.99) |
| Group II | 15/41 (36.6) | 1.00 | 1.00 | |
| Group III | 21/34 (61.8) | 2.95 (1.40-6.24)b | 2.98 (1.29-6.87)a | |
| (P trend < 0.05) | (P trend: NS) | |||
| Group (I+II) | 26/73 (35.6) | 1.00 | 1.00 | |
| Group III | 21/34 (61.8) | 2.44 (1.33-4.48)d | 2.36 (1.17-4.78)c | |
Group III had the highest rate of Child A to B progression (21 of 34, 61.8%), followed by <3.5 albumin (50.0%), and <100 PT (48.8%) (Table 2). A significant relationship was found between group (I+II) and group III (crude RR = 2.44, 95%CI = 1.33-4.48, P < 0.01), and between group II and group III (2.95, 1.40-6.24, P < 0.01), with a trend of significance (P < 0.05). No significant increase of other predictors was revealed. PSI and the common useful predictors such as albumin and platelets were included in multivariate analysis; only group III remained significant (adjusted RR = 2.98, 95%CI = 1.29-6.87, P < 0.05).
The group with < 10 platelets had the highest incidence of HCC and the highest progression of varices (30.3%, 47.1%, respectively). On multivariate analyses, no significant associations were found between PSI and incidence of HCC or progression of esophagogastric varices.
Even if physical symptoms and serum biochemical tests indicate the early phase of LC-C, the patient may already have occult advanced hepatic damage. PSI is a possible predictor of occult progressive stages of LC-C for outcome patients. While PSI obtained by per-rectal portal scintigraphy has its own weaknesses (it emphasizes PSS via the inferior mesenteric vein, rather than via the superior mesenteric vein, and expresses the extent of PSS indirectly), it should be useful for the observation of LC-C because it is a simple and non-invasive technique unlike hepatic venous catheterization[9].
In this study, we used 99mTc pertechnetate for per-rectal portal scintigraphy because of its short half-life and low cost[17]. Our study had three major findings.
First, there was no correlation between the porto-systemic hypertension and HCC incidence. This finding suggests that HCC occurs independently of the decrease in hepatic blood flow due to the development of PSS.
Second, patients with LC-C of Child A will progress to Child B rapidly after their PSI reaches 30% or higher. Shiomi et al[5] have reported that changes in the portal hemodynamics of chronic liver disease subjects were not gradual. The development of PSS causes hepatic functional reserve to deteriorate rapidly. We propose that per-rectal portal scintigraphy is useful to predict occult progressive portal hypertension and liver failure in the early phase of LC-C, on the basis of the strong relationship between PSI and the Child-Pugh staging.
Third, the natural advance of PSS has relevance to esophagogastric varices progression in patients with the early phase of LC-C. Other authors have reported that the porto-systemic pressure gradient is a strong predictor for varices progression[18,19]. But in this study, PSI showed no statistical advantage over platelets, albumin, or T-bil for detecting the progression of varices. The reason why PSI was worse than these laboratory data is because esophagogastric varices mainly reflect the flow of superior mesenteric vein.
Progressive viral hepatitis has been acknowledged as a major indication for liver transplantation[20,21]. Kiuchi et al[22] have emphasized the need to evaluate the recipients preoperatively. One of the important recipient factors is the presence of collateral circulation[23]. Bruix et al[24] have reported that LC patients with increased portal pressure are at high risk of hepatic decompensation after resection of HCC. We propose that preoperative per-rectal portal scintigraphy would be useful for early detection of occult portal hypertension, to assess graft size requirement to prevent graft failure after liver transplantation, or to avoid liver failure after hepatectomy.
In summary, physicians can monitor the porto-systemic hypertension gradient in LC patient during the outcome observation period by using “non-invasive” per-rectal portal scintigraphy; on the other hand, measurement of HVPG needs hospitalization. In the early phase of LC-C, PSI can be used to predict occult progressive PSS and liver failure. Therefore, even for patients diagnosed as being in the early phase of LC-C on the basis of other indicators, those with an initial PSI ≥ 30% should be observed by keeping early liver transplantation, or liver failure after hepatectomy in mind; HCC should be watched for, regardless of PSI.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, Schleck CD, Zinsmeister AR. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | Fattovich G, Zagni I, Minola E, Felder M, Rovere P, Carlotto A, Suppressa S, Miracolo A, Paternoster C, Rizzo C. A randomized trial of consensus interferon in combination with ribavirin as initial treatment for chronic hepatitis C. J Hepatol. 2003;39:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Iino S. Natural history of hepatitis B and C virus infections. Oncology. 2002;62 Suppl 1:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Shiomi S, Sasaki N, Habu D, Takeda T, Nishiguchi S, Kuroki T, Tanaka T, Ochi H. Natural course of portal hemodynamics in patients with chronic liver diseases, evaluated by per-rectal portal scintigraphy with Tc-99m pertechnetate. J Gastroenterol. 1998;33:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Debernardi-Venon W, Bandi JC, García-Pagán JC, Moitinho E, Andreu V, Real M, Escorsell A, Montanyá X, Bosch J. CO(2) wedged hepatic venography in the evaluation of portal hypertension. Gut. 2000;46:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 8. | Patch D, Nikolopoulou V, McCormick A, Dick R, Armonis A, Wannamethee G, Burroughs A. Factors related to early mortality after transjugular intrahepatic portosystemic shunt for failed endoscopic therapy in acute variceal bleeding. J Hepatol. 1998;28:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Shiomi S, Kuroki T, Kurai O, Kobayashi K, Ikeoka N, Monna T, Ochi H. Portal circulation by technetium-99m pertechnetate per-rectal portal scintigraphy. J Nucl Med. 1988;29:460-465. [PubMed] |
| 10. | Bołdys H, Hartleb M, Rudzki K, Nowak A, Nowak S. Effect of propranolol on portosystemic collateral circulation estimated by per-rectal portal scintigraphy with technetium-99m pertechnetate. J Hepatol. 1995;22:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5736] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 12. | Kusaka K, Harihara Y, Torzilli G, Kubota K, Takayama T, Makuuchi M, Mori M, Omata S. Objective evaluation of liver consistency to estimate hepatic fibrosis and functional reserve for hepatectomy. J Am Coll Surg. 2000;191:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995;19:420-442; discussion 423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Schlichting P, Christensen E, Andersen PK, Fauerholdt L, Juhl E, Poulsen H, Tygstrup N. Prognostic factors in cirrhosis identified by Cox's regression model. Hepatology. 1983;3:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Holford TR. Life tables with concomitant information. Biometrics. 1976;32:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 89] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Meijer K, Haagsma EB, Kok T, Schirm J, Smid WM, van der Meer J. Natural history of hepatitis C in HIV-negative patients with congenital coagulation disorders. J Hepatol. 1999;31:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Urbain D, Jeghers O, Ham HR. Per-rectal portal scintigraphy: comparison between technetium-99m, thallium-201, and iodine-123-HIPDM. J Nucl Med. 1988;29:2020-2021. [PubMed] |
| 18. | Vorobioff J, Groszmann RJ, Picabea E, Gamen M, Villavicencio R, Bordato J, Morel I, Audano M, Tanno H, Lerner E. Prognostic value of hepatic venous pressure gradient measurements in alcoholic cirrhosis: a 10-year prospective study. Gastroenterology. 1996;111:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 213] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407. [PubMed] |
| 20. | Villeneuve JP, Durantel D, Durantel S, Westland C, Xiong S, Brosgart CL, Gibbs CS, Parvaz P, Werle B, Trépo C. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol. 2003;39:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Rosen HR, Gretch D, Kaufman E, Quan S. Humoral immune response to hepatitis C after liver transplantation: assessment of a new recombinant immunoblot assay. Am J Gastroenterol. 2000;95:2035-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, Ogawa K. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Fevery J. Liver transplantation: problems and perspectives. Hepatogastroenterology. 1998;45:1039-1044. [PubMed] |
| 24. | Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 621] [Article Influence: 21.4] [Reference Citation Analysis (0)] |