INTRODUCTION
In 1998, star-shaped cells in the pancreas, namely pancreatic stellate cells (PSCs), were identified and characterized[1,2]. In normal pancreas, stellate cells are quiescent and can be identified by the presence of vitamin A-containing lipid droplets in the cytoplasm. In response to pancreatic injury or inflammation, they are transformed (“activated”) from their quiescent phenotype into myofibroblast-like cells which actively proliferate, express α-smooth muscle actin, and produce type I collagen and other extracellular matrix components. There is accumulating evidence that activated PSCs play a pivotal role in the development of pancreatic fibrosis[1-4]. It has also been suggested that PSCs may participate in the pathogenesis of acute pancreatitis[3,5]. The molecular mechanisms responsible for PSC activation remain to be elucidated, but the activation of signaling pathways such as p38 mitogen-activated protein (MAP) kinase[6], Rho-Rho kinase pathway[7], and c-Jun N-terminal kinase[8] is likely to play a role. However, intracellular signaling pathways in PSCs remain largely unknown.
Stellate cell proliferation and expansion of their pool are a fundamental feature of pancreatic fibrosis[3]. Platelet-derived growth factor (PDGF) is one of the most potent mitogen of PSCs, and is likely to be an important mediator of the increased proliferation of the cells both in vivo and in vitro[9,10]. PDGF is a polypeptide growth factor that exists as a disulfide-linked homodimer (PDGF-AA or -BB) or a heterodimer (PDGF-AB) of two chains, A or B[11,12]. Two PDGF receptor subtypes bind to the three isoforms of PDGF differentially; PDGF β-receptor can interact only with B-chain containing isoforms whereas PDGF α-receptor can bind to all three isoforms. Binding of the ligands to the receptors leads to dimerization of receptor subunits, phosphorylates itself on tyrosines (known as “autophosph-orylation”), changes its cytoplasmic conformation, activates endogenous tyrosine phosphorylating activity, and initiates intracellular signaling[11,12]. It has been shown that activation of extracellular signal-regulated kinase (ERK) plays a critical role in PDGF-induced proliferation of PSCs[13,14]. But, the fact that activation of ERK was required though not sufficient for PSC proliferation[15] suggests that other signaling pathways might be involved. Janus-activated kinases (JAKs) are a group of non-receptor tyrosine kinases that, via phosphorylation, modulate the activities of a group of transcription factors, viz. signal transducers and activators of transcription (STAT)[16,17]. The STAT proteins exist in a latent form in the cytoplasm, and, upon receptor activation by cytokines and growth factors, become phosphorylated on tyrosine residues[16,17]. This phosphorylation results in translocation of the STATs to the nucleus, where they bind to sequence-specific DNA elements[16]. Recent studies have shown that activation of JAK2-STAT3 pathway mediates PDGF-induced proliferation in several types of cells such as NIH3T3 fibroblasts[18], human airway smooth muscle cells[19], and rat vascular smooth muscle cells[20]. But, possible regulation of cell functions by JAK-STAT pathway in PSCs is unknown.
In this study, we examined the role of JAK-STAT pathway in PSC proliferation in response to PDGF-BB. We report here that PDGF-BB activated JAK2-STAT3 pathway via Src-dependent mechanism, and that activation of this pathway is required for PDGF-induced PSC proliferation.
MATERIALS AND METHODS
Materials
Poly(dI.dC)-poly(dI.dC) and [γ-32P]ATP were purchased from Amersham Biosciences UK, Ltd. (Buckinghamshire, UK). Rat recombinant PDGF-BB was obtained from R&D Systems (Minneapolis, MN, USA). Rabbit antibodies against phosph-orylated Src, phosphorylated JAK2, STAT1 (phosphorylated at Tyr701 and total), STAT3 (phosphorylated at Tyr705 and total), and ERK (phosphorylated and total) were purchased from Cell Signaling Technology Inc. (Beverly, MA). Rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Trevigen (Gaithersburg, MD). AG490 and U0126 were purchased from Calbiochem (La Jolla, CA). PP1 and rabbit antibody against phosphorylated PDGF β-receptor were obtained from Upstate Biotechnology Inc. (Lake Placid, NY). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specifically described.
Cell culture
All animal procedures were performed in accordance with the National Institutes of Health Animal Care and Use Guidelines. Rat PSCs were prepared from the pancreas tissues of male Wistar rats (Japan SLC Inc., Hamamatsu, Japan) weighing 200-250 g as previously described using the Nycodenz solution (Nycomed Pharma, Oslo, Norway) after perfusion with 0.3 g/L collagenase P[14]. The cells were resuspended in Ham’s F-12 containing 100 mL/L heat-inactivated fetal bovine serum (ICN Biomedicals, Aurora, OH), penicillin sodium, and streptomycin sulfate. Cell purity was always more than 90% as assessed by a typical star-like configuration and by detecting vitamin A autofluorescence. All experiments were performed using cells between passages two and five. Unless specifically described, we incubated PSCs in serum-free medium for 24 h before the addition of experimental reagents.
Immunostaining
Serum-starved PSCs were grown directly on slides, and immunostaining for PDGF β-receptor was performed as previously described using a streptavidin-biotin-peroxidase complex detection kit (Histofine Kit; Nichirei, Tokyo, Japan). Briefly, cells were fixed with 1000 mL/L methanol for 10 min at -20 °C, and then endogenous peroxidase activity was blocked by incubation in methanol with 3 mL/L hydrogen peroxide for 5 min. After immersion in normal goat serum for 1 h, the slides were incubated with rabbit anti-PDGF β-receptor antibody overnight at 4 °C. The slides were incubated with biotinylated goat anti-rabbit Ig antibody for 45 min, followed by peroxidase-conjugated streptavidin for 30 min. Finally, color was developed by incubating the slides for several min with diaminobenzidine (Dojindo, Kumamoto, Japan). For a negative control, the primary antibody was replaced with non-immune rabbit IgG.
Western blotting
Western blotting was performed as previously described[21]. Cells were treated with PDGF-BB and lysed in sodium dodecyl sulfate (SDS) buffer. Cellular proteins (approximately 100 μg) were fractionated on a 100 g/L SDS-polyacrylamide gel, and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). The level of activated, phosphorylated STAT3 was determined by Western blotting using anti-phosphospecific STAT3 antibody. The antibody recognizes only phosphor-ylated form of STAT3, thus allowing the assessment of activation of the kinase. After incubation with a secondary antibody (goat anti-rabbit, horseradish peroxidase conjugated), proteins were visualized by using an ECL kit (Amersham Biosciences UK, Ltd.). The levels of total STAT3, phospho-rylated PDGF β-receptor, phosphorylated Src, phosphorylated JAK2, STAT1 (phosphorylated and total), ERK (phosphorylated and total), and GAPDH were also determined in a similar manner.
Nuclear extracts preparation and electrophoretic mobility shift assay
Nuclear extracts were prepared after 15 min treatment, and electrophoretic mobility shift assay was performed as previously described[22]. Double-stranded STAT consensus oligonucleotide probe m67 (5’-CATTTCCCGTAAATC-3’) was endlabeled with [γ-32P]ATP. m67 is a high-affinity mutant of the sis-inducible element of the human c-fos gene, and binds to STAT1 and STAT3[23]. Nuclear extracts (approximately 5 μg) were incubated with the labeled oligonucleotide probe for 20 min at 22 °C, and electrophoresed through a 40 g/L polyacrylamide gel. Gels were dried, and autoradiographed at -80 °C overnight. A 100-fold excess of unlabeled oligonu-cleotide was incubated with nuclear extracts for 10 min prior to the addition of the radiolabeled probe in the competition experiments. For super shift assays, nuclear extracts were incubated for 1 h at 4 °C with antibodies against STAT1 or STAT3 before incubation with the radiolabeled probe.
Cell proliferation assay
Cell proliferation was assessed using a commercial kit (Cell proliferation ELISA, BrdU; Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instruction. This is a colorimetric immunoassay based on the measurement of 5-bromo-2’-deoxyuridine (BrdU) incorporation during DNA synthesis. After 24-h incubation with experimental reagents, cells were labeled with BrdU for 3 h at 37 °C. Cells were then fixed, and incubated with peroxidase-conjugated anti-BrdU antibody. Then the peroxidase substrate 3,3’,5,5’-tetramethylbenzidine was added, and BrdU incorporation was quantitated by A370-492.
Antisense oligonucleotide transfection
The antisense, phosphorothioated 21-mer oligonucleotides directed against the translation start site (AUG codon) and surrounding nucleotides of the rat STAT1 gene and STAT3 genes were synthesized by SigmaGenosys, Ishikari, Japan. The STAT1 antisense oligonucleotide sequence was 5’-CCACTGCGACATCCTCTTTAA-3’ and the corresponding sense oligonucleotide sequence was 5’-TTAAAGAGGAT-GTCGCAGTGG-3’. The STAT3 antisense oligonucleotide sequence was 5’-CCACTGAGCCATCCTGCCGCA-3’ and the corresponding sense oligonucleotide sequence was 5’-TGCGGCAGGAYGGCTCAGTGG-3’. Serum-starved PSCs were treated with sense, antisense, or scramble oligonucleotides (5’-CAAATGGGCTCCGACGTCGGT-3’) in the presence of FUGENE6 (Roche Diagnostics). After 24 h, cells were stimulated with PDGF-BB (at 25 ng/mL) for another 24 h, and proliferation was assessed by the incorporation of BrdU as described before.
Statistical analysis
The results were expressed as mean±SD. Luminograms and photographs are representative of at least three experiments. Differences between the groups were evaluated by ANOVA, followed by Fisher’s test for post hoc analysis. A P-value less than 0.05 was considered statistically significant.
RESULTS
Activated PSCs expressed PDGF-receptor
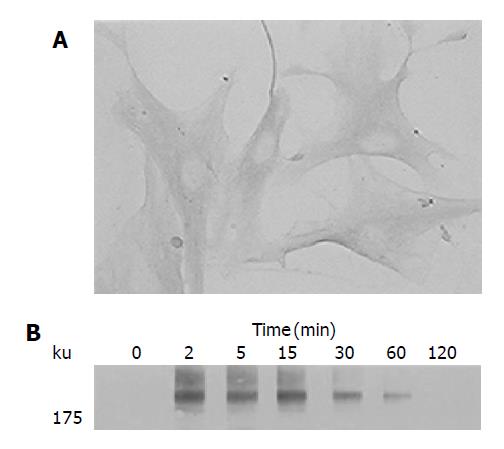
We first examined the expression of PDGF-receptor in PSCs by immunostaining. Culture-activated PSCs expressed PDGF β-receptor (Figure 1A). Negative control using non-immune rabbit IgG in place of the specific antibody gave little staining (data not shown). The PDGF receptors dimerize, and become autophosphorylated on tyrosine residues upon binding to their ligands[12]. Indeed, PDGF-BB induced tyrosine phosphorylation of the PDGF β-receptor in a time-dependent manner (Figure 1B). In this study, the isoform PDGF-BB was selected for use because the previous study reported that PDGF-AA had little effect on PSC proliferation[9].
Figure 1 Activated PSCs expressed PDGF β-receptor.
A: Serum-starved, culture-activated PSCs were grown directly on slides. Immunostaining for PDGF β-receptor was performed using a streptavidin-biotin-peroxidase complex detection kit. Original magnification: ×20 objective; B: Serum-starved PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates were prepared, and separated by 70 g/L SDS-polyacrylamide gel electrophoresis. The phosphorylation of PDGF β-receptor was examined by Western blotting.
PDGF-BB activated STAT1 and STAT3
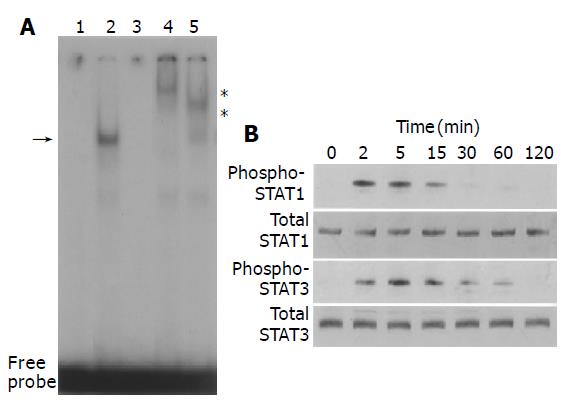
We examined whether PDGF-BB activated STAT pathway. PDGF-BB increased STAT-specific DNA binding activity as assessed by electrophoretic mobility shift assay (Figure 2A). The specificity of the DNA binding activity was demonstrated by the addition of a 100-fold molar excess of unlabeled STAT oligonucleotide, and not by unrelated Oct-1 oligonucleotide in competition assays (Figure 2A, data not shown). Specific antibodies against STAT1 and STAT3 retarded the DNA-protein complex, indicating that the complex consisted of STAT1 and STAT3. We also examined whether PDGF-BB activated STAT1 and STAT3 by Western blotting using anti-phosphospecific antibodies. PDGF-BB activated STAT1 and STAT3 in a time-dependent manner, with peaking around 5-15 min (Figure 2B).
Figure 2 PDGF activated STAT1 and STAT3.
A: PSCs were treated with PDGF-BB (at 25 ng/mL, lane 2) in serum-free medium for 15 min. Nuclear extracts were prepared and subjected to electrophoretic mobility shift assay using STAT consensus oligonucleotide probe m67. Arrow denotes specific inducible complex competitive with cold double-stranded oligonucleotide probe (lane 3). For super shift assays, nuclear extracts were incubated with antibodies against STAT1 (lane 4) or STAT3 (lane 5) before incubation with the radiolabeled probe. *: super shifts. Lane 1: control (serum-free medium only); B: PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of STAT1 and STAT3 was examined by Western blotting using anti-phosphospecific antibodies. The levels of total STAT1 and STAT3 were also determined.
Src and JAK2 mediate activation of STAT1 and STAT3
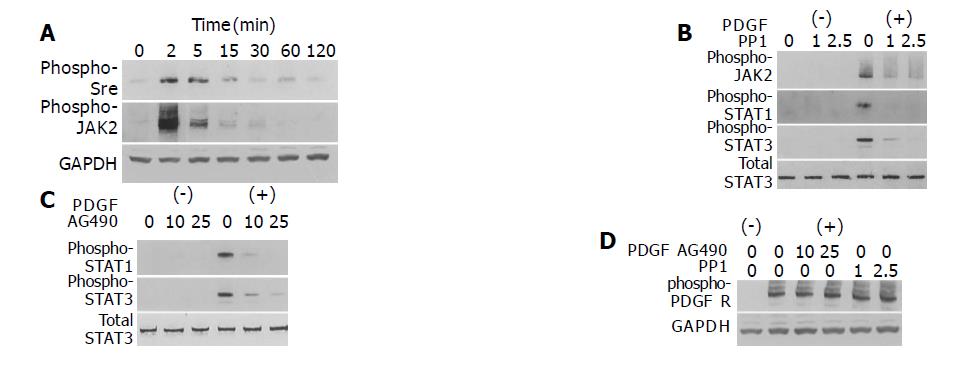
The mechanism of growth factor-induced activation of STAT is not fully elucidated; JAK, Src, and receptor tyrosine kinases might all be involved[24]. PDGF-BB induced phosphorylation of Src and JAK2 in a time-dependent manner (Figure 3A). To determine which kinase(s) mediates PDGF-induced activation of STAT1 and STAT3, we employed specific inhibitors of Src and JAK2. Pretreatment of PSCs with a specific Src inhibitor PP1[25] inhibited STAT1 and STAT3 activation as well as that of JAK2 (Figure 3B). Pretreatment of PSCs with AG490, a specific JAK2 kinase inhibitor[26], also blocked PDGF-BB-induced STAT1 and STAT3 activation (Figure 3C). These inhibitory effects were not due to the cytotoxicity, because these reagents did not affect the viability assessed by trypan blue dye exclusion test (data not shown). Neither AG490 nor PP1 inhibited autophosphorylation of the PDGF β-receptor (Figure 3D). Thus, the activation of STAT1 and STAT3 by PDGF-BB required the activation of both JAK and Src, and Src was located upstream of JAK for PDGF signaling in PSCs.
Figure 3 Src and JAK2 mediate the activation of STAT1 and STAT3.
A: PSCs were treated with PDGF-BB (at 25 ng/mL) for the indicated time. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of Src and JAK2 was examined by Western blotting using anti-phosphospecific antibodies. The level of GAPDH was also determined; B-D: PSCs were treated with a Src inhibitor PP1 (at 1 or 2.5 μmol/L) or a JAK2 inhibitor AG490 (at 10 or 25 μmol/L) in the absence or presence of PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates (approximately 100 μg) were prepared, and the levels of phosphorylated JAK2, STAT1, STAT3, and PDGF β-receptor were determined by Western blotting. The levels of total STAT3 and GAPDH were also determined.
Ethanol and acetaldehyde decreased basal activation of JAK2 and STAT3
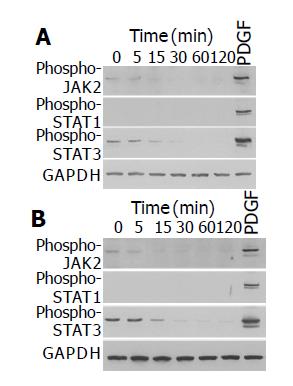
We have previously shown that ethanol and acetaldehyde at clinically relevant concentrations activated ERK, but did not induce proliferation of PSCs[15]. We examined whether ethanol and acetaldehyde activated JAK-STAT pathway. Ethanol and acetaldehyde at clinically relevant concentrations (50 mmol/L and 200 mmol/L, respectively) decreased basal activation of JAK2 and STAT3 in a time-dependent manner (Figure 4). The basal activation of STAT1 could not be detected even after long exposure.
Figure 4 Ethanol and acetaldehyde decreased basal activation of JAK2 and STAT3.
PSCs were treated with ethanol (at 50 mmol/L, panel A) or acetaldehyde (at 200 mmol/L, panel B) for the indicated time, or with PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates (approximately 100 μg) were prepared, and separated by 100 g/L SDS-polyacrylamide gel electrophoresis. The activation of JAK2, STAT1, and STAT3 was examined by Western blotting using anti-phosphospecific antibodies. The level of GAPDH was also determined.
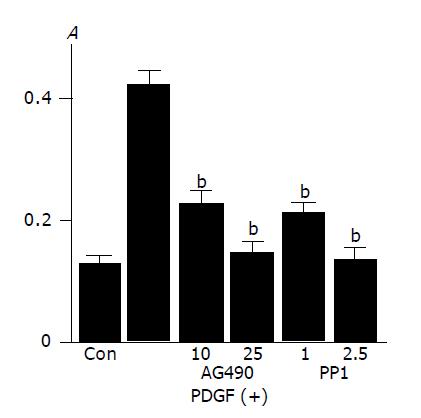
Activation of Src, JAK2, and STAT3 is required for PDGF-BB-induced proliferation
In agreement with the previous studies showing that PDGF-BB is a potent mitogen for PSCs[9,10], PDGF-BB increased PSCs proliferation as assessed by BrdU incorporation (Figure 5A). This increase in BrdU incorporation was inhibited by AG490 and PP1, suggesting that JAK2 and Src kinases are required for proliferation.
Figure 5 Activation of Src and JAK2 is required for PDGF-induced proliferation and serum-starved PSCs were treated with PDGF-BB (at 25 ng/mL) in the presence or absence of AG490 (at 10 or 25 μmol/L) or PP1 (at 1 or 2.
5 μmol/L). After 24-h incubation, DNA synthesis was assessed by BrdU incorporation ELISA. Data are shown as mean±SD (n = 6). bP<0.01 versus PDGF-BB only. Con: control (serum-free medium only), A: optical density.
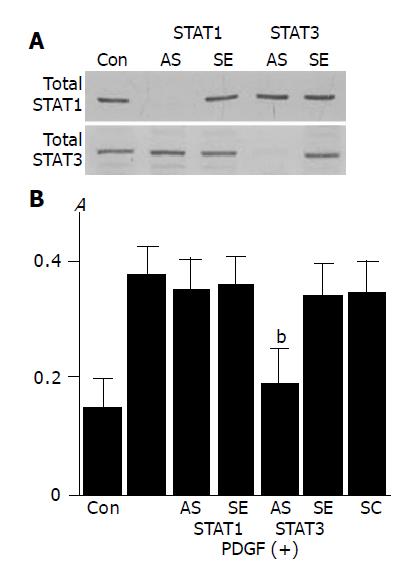
To clarify the role of STAT1 and STAT3 in PDGF-BB-induced proliferation we employed STAT1 and STAT3 antisense oligonucleotides. STAT1 and STAT3 antisense oligonucleotides directed against their translation initiation sites prevented the synthesis of STAT1 and STAT3, respectively (Figure 6A). PDGF-induced proliferation was abolished by the transfection of STAT3 antisense oligonucleotide, and not of STAT1 antisense oligonucleotide (Figure 6B).
Figure 6 Activation of STAT3, and not STAT1, is required for PDGF-induced proliferation.
A: PSCs were treated with antisense (“AS”) or sense (“SE”) oligonucleotides for STAT1 or STAT3 for 24 h. The expression of total STAT1 and STAT3 was examined by Western blotting; B: PSCs were treated with sense, antisense, or scramble (“SC”) oligonucleotides. After 24 h, cells were stimulated with PDGF-BB (at 25 ng/mL) for another 24 h, and DNA synthesis was assessed by BrdU incorporation enzyme-linked immunosorbent assay. Data are shown as mean±SD (% of the control, n = 6). bP<0.01 vs PDGF-BB only. Con: control (serum-free medium only), A: optical density.
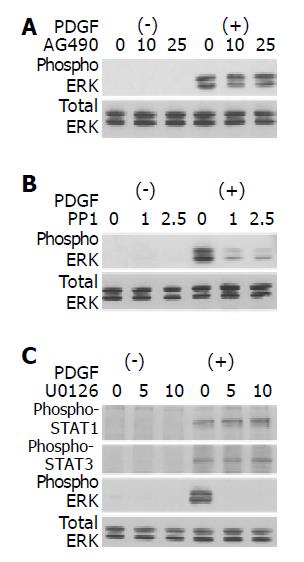
Src, not JAK2, is located upstream of ERK
It has been reported that activation of ERK pathway plays critical roles in PDGF-induced proliferation of PSCs[13,14]. We examined whether PP1 and AG490 inhibited PSC proliferation through the inhibition of ERK activation. AG490 did not affect PDGF-BB-induced activation of ERK (Figure 7A). PP1, however, did inhibit PDGF-BB-induced activation of ERK (Figure 7B). On the other hand, U0126, an inhibitor of MAP kinase kinase and consequent ERK activation[27], did not affect the activation of STAT1, and STAT3 (Figure 7C). Thus, ERK and JAK2-STAT3 pathways exert independent regulatory effects on PDGF-induced proliferation in PSCs.
Figure 7 Src, not JAK2, is located upstream of ERK.
PSCs were treated with a JAK2 inhibitor AG490 (A) (at 10 or 25 μmol/L), a Src inhibitor PP1 (B) (at 1 or 2.5 μol/L), or a MAP kinase kinase inhibitor U0126 (C) (at 5 or 10 μmol/L) in the absence or presence of PDGF-BB (at 25 ng/mL) for 5 min. Total cell lysates were prepared, and the levels of phosphorylated ERK, STAT1, and STAT3 were determined by Western blotting. The level of total ERK was also determined.
DISCUSSION
Stellate cell proliferation and expansion of their pool are a fundamental feature of pancreatic fibrosis[3]. PDGF-BB has been shown to be one of the most potent mitogens of PSCs in vitro[9,10]. It has been reported that activation of ERK pathway plays a critical role in PDGF-induced proliferation of PSCs[13,14]. On the other hand, we have previously reported that ethanol and acetaldehyde at clinically relevant concentrations activated ERK, but failed to induce proliferation in PSCs[15], suggesting that other signaling pathways might be involved in this process as well. In this study, we examined the role of JAK-STAT pathway in PDGF-induced proliferation of PSCs. We showed here that PDGF induced the activation of Src, JAK2, STAT1, and STAT3 in PSCs. JAK proteins phosphorylate STAT proteins at tyrosine residues and activate them, although other mechanisms might be involved[28,29]. In PSCs, the activation of STAT1 and STAT3 was inhibited by a Src kinase inhibitor PP1 and a JAK2 kinase inhibitor AG490, suggesting that the activation of STAT was dependent on Src and JAK2. PDGF-induced proliferation was inhibited by PP1, AG490, and STAT3 antisense oligonucleotide, and not by STAT1 antisense oligonucleotide. Thus, activation of Src-JAK2-STAT3 pathway plays a role in PDGF-induced proliferation in PSCs. Although STAT pathway has been shown to play a role in proliferative responses in some cell types[18-20], no previous studies have examined whether STAT pathway is activated and it regulates cell functions in PSCs. Detailing STAT signaling in PSCs is important because regulation and functions of STATs differ greatly depending on the cell type. Our results are in agreement with the previous studies showing a role of this pathway in the regulation of cell growth in several cell types including NIH3T3 fibroblasts[18], human airway smooth muscle cells[19], and rat vascular smooth muscle cells[20].
We showed here that culture-activated PSCs expressed PDGF β-receptor. During tissue repair and inflammatory processes in the pancreas, PDGF is secreted by various cells including platelets, mononuclear cells, and activated macrophages[30]. Exposure of PSCs to PDGF in vivo is likely to occur in conditions of pancreatic inflammation characterized by the presence of platelets and activated macrophages[2,10]. PDGF-induced effects on PSCs in vivo may be further aided by the upregulation of PDGF receptors in PSCs. In this regard, it is of interest to note that in a rat model of pancreatic fibrosis, immunostaining for PDGF β-receptor was found to be increased in activated PSCs in the area of fibrosis[3]. Codistribution of PDGF with cells expressing its receptor confirms a functional role of PDGF in the development of pancreatic fibrosis.
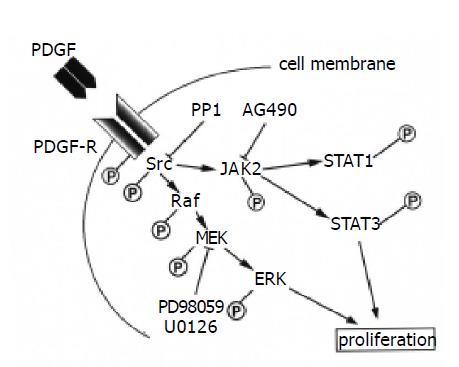
In addition to ERK[13,14], this study indicates that the Src-JAK2-STAT3 pathway is also required for proliferation in PSCs. The STAT pathway has been shown to interact with MAP kinase pathway in different cellular context. For example, the STAT transcription factors are a potential convergence site for the MAP kinase and JAK kinase pathways; JAKs phosphorylate STATs on tyrosine, leading to dimerization and nuclear localization, whereas MAP kinase phosphorylates STAT1 and STAT3 on serine, leading to maximal transcriptional activation[17,31]. Considering that PDGF activates multiple signaling pathways, including phosphatidylinositol 3-kinase and the MAP kinase pathway in PSCs[13,14], it is possible that Src-JAK2-STAT3 pathway cooperates with these signaling pathways in mediating the proliferative response in PSCs. Inhibition of Src by PP1 blocked the activation of ERK as well as of JAK2-STAT3, suggesting that Src is located upstream of ERK. This is consistent with the previous report that Src kinase is upstream of ERK activation and can participate in Raf activation[32]. Although AG490 was effective in blocking STAT3 activation and proliferation, it did not have any apparent effect on PDGF-induced ERK activation. On the other hand, inhibition of ERK by U0126 abolished PDGF-induced proliferation, but not the activation of JAK2 and STAT3. Collectively, Src is a potential convergence site for the MAP kinase and JAK-STAT pathway, and each pathway may exert independent regulatory effects on PDGF-induced proliferation in PSCs (Figure 8).
Figure 8 Signaling pathways for PDGF-induced proliferation in PSCs.
Although it has been shown that PDGF activates STAT1 in hepatic stellate cells[33], no previous studies have addressed the role of JAK-STAT pathway in the proliferative response in PSCs or hepatic stellate cells. We showed here that PDGF activated STAT1 and STAT3 in PSCs. Although STAT3 appears to play a role in proliferation in PSCs, the responsible mechanisms for STAT3-mediated proliferation remain obscure. Potential STAT target genes for mediating PDGF-induced proliferation of PSCs include the immediate-early gene c-myc, the cell cycle regulatory gene cyclin D1, and the anti-apoptotic genes Bcl-2 and Bcl-x[34]. In vascular smooth muscle cells, it has been recently shown that induction of cytosolic phospholipase A2 expression and arachidonic acid release are involved in PDGF-BB-stimulated cell growth and that these events are mediated by JAK2-dependent STAT3 activation[20]. Along this line, PDGF-induced PSC proliferation was blocked by AACOCF3, an inhibitor of cytosolic phospholipase A2 (Masamune et al, unpublished observation).
We have previously shown that ethanol and acetaldehyde at clinically relevant concentrations activated ERK, but did not induce proliferation of PSCs[15]. We showed here that, unlike PDGF, ethanol and acetaldehyde did not induce the activation of JAK-STAT pathway. Interestingly, ethanol and acetaldehyde decreased basal activation of JAK2 and STAT3 in PSCs. This is in agreement with the previous study showing that ethanol inhibited the activation of STAT3 in rat hepatocytes[35]. It is possible that failure of proliferation induction by ethanol and acetaldehyde may be attributable to the failure of JAK-STAT pathway activation. Obviously, further studies are required to test this hypothesis.
It remains unknown as to the role of STAT1 in the cell functions of PSCs. In addition to its roles in cell immunity, proliferation, apoptosis, and transformation, recent studies have suggested that STAT1 participates in the development of fibrosis. For example, STAT1 mediates transforming growth factor-β and fibronectin synthesis in response to high glucose in glomerular mesangial cells[36]. STAT1 as well as STAT3 mediate thrombin-induced upregulation of tissue type inhibitor metalloproteinase-1 in human glomerular mesangial cells[37]. Interestingly, Weiskirchen et al, reported that LIM-domain protein cysteine- and glycine-rich protein 2 (CRP2) is a potential new factor in the JAK-STAT pathway in hepatic stellate cells[38]. The protein inhibitor of activated STAT1 was shown to be selectively associated with the C-terminal LIM domain of CRP2, and the suppression of CRP2 might be a prerequisite for the myofibroblastic transition of hepatic stellate cells. Further studies are necessary to clarify the role of altered expression of STAT1 and CRP2 in the activation process of PSCs.
In summary, we have shown that PDGF activated STAT1 and STAT3, and that the activation of JAK2-STAT3 pathway plays a role in the PDGF-induced proliferation of PSCs. A better understanding of the molecular mechanisms of PSC proliferation may provide more rational targets for the treatment of patients with pancreatic fibrosis and inflammation.