Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3304
Revised: May 26, 2004
Accepted: June 24, 2004
Published online: June 7, 2005
AIM: To investigate the mutation in D-loop region of mitoc-hondrial DNA in gastric cancer and its influence on the changes of reactive oxygen species (ROS) and cell cycle.
METHODS: The D-loop region was amplified by PCR and sequenced. Reactive oxygen species and cell cycle were detected by flow cytometry in 20 specimens from gastric cancer and adjacent normal tissues. According to the sequence results, gastric cancer tissue was divided into mutation group and control group. Reactive oxygen species, apoptosis and proliferation in the two groups were compared.
RESULTS: Among the 20 gastric cancer specimens, 18 mutations were identified in 7 patients, the mutation rate being 35%. There were four microsatellite instabilities in the mutations. No mutation was found in the adjacent tissues. Reactive oxygen species, apoptosis, and proliferation in the mutation group were all significantly higher than those in control group.
CONCLUSION: Mutation in D-loop region plays a role in the genesis and development of gastric cancer.
- Citation: Zhao YB, Yang HY, Zhang XW, Chen GY. Mutation in D-loop region of mitochondrial DNA in gastric cancer and its significance. World J Gastroenterol 2005; 11(21): 3304-3306
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3304
The occurrence and progression of gastric cancer are a multi-step process due to multiple factors and gene mutations. Although great success has been achieved in oncogenes and cancer suppressor genes, a lot of questions cannot be explained by alteration of nuclear genes. In recent years, mutation of mitochondrial DNA (mtDNA) has been associated with the occurrence and progression of tumor and is regarded as a possible causitive factor for cancer[1].
Human mtDNA is a closed double strand circular molecule of 16569 bp, coding 13 proteins, 22 tRNAs and 2 rRNAs. Its D-loop is a non-coding region, containing some important sequences such as the promoter for heavy chain replication. Because of the properties of its structure as well as its mechanism of DNA replication and injury repair, the mutation frequency of mitochondria is 10-100×higher than that of nuclear DNA. In recent years, the instability, gene mutation or abnormal expression of mitochondrial genome has been detected in many kinds of malignant cancer tissues[2,3]. The mitochondrial D-loop is a hotspot for gene mutation in cell lines of colonic and rectal cancer. But there is a difference in mutation frequency of the D-loop among different tumors[4-7].
In this report the region of mtDNA D-loop was amplified by PCR and then sequenced with samples from cancer tissues and corresponding normal mucous membrane of 20 gastric cancer patients. Reactive oxygen species (ROS) and cell cycle were detected with flow cytometry. The samples were divided into mutation group and control group according to the mutations. We compared the level of ROS and cell cycle within two groups to clarify the influence of mutations of D-loop on ROS and cell cycle. Our purpose was to investigate the influence of mutation of D-loop on cell carcinogenesis and progression of gastric cancer.
Twenty surgical samples of gastric cancer were selected from hospitalized patients in the Department of Gastroenterology Surgery of Jiangsu Provincial Hospital from May to November, 2003. All patients, 13 males and 7 females, were diagnosed by gastroscopy and biopsy before operation. Their ages varied from 39 to 78 years and averaged 57.2 years.
A region without bleeding was carefully selected. A block of 1.0 cm3 fresh cancer tissue was cut and stored in a -70 °C refrigerator. Other tissues were cleaned with PBS. Cell suspension was prepared by mechanical grinding and filtered through a net and cancer cells were separated by centrifugation (proved by Rye’s dyeing). A block of 1.0 cm3 normal gastric tissue was cut and cell suspension was prepared according to the same procedure.
Half of 1 mL cell suspension was put into a test tube, centrifuged for 5 min at 1500 r/min, washed thrice with normal saline water and cell debris were by centrifugation for 3 min at 500-800 r/min. Cells were fixed with 2-4 mL 1% poly-formaldehyde and centrifuged for 10 min at 1500 r/min. The supernate was discarded and the pallet was resuspended in 2-4 mL 0.1% Triton-X-100 for 3 min and centrifuged. The supernate was discarded and resuspended in 1-2 mL 0.01% RNase, vortexed for 30 min in 37 °C water bath. One milliliter 0.05% PI solution was added to dye the DNA for 30 min. The cell cycle of cancer cells and normal gastric cells was measured by FACS (Vantage SE, BD, USA). The fluorescence signal was processed by multicycle analytical software for cell cycle.
DCFH-DA (from Sigma Company) was dissolved in 95% ethanol to a concentration of 5 mmol/L and stored at 4 °C in the dark, diluted to 5 μmol/L with PBS before use.
Two hundred microliter cell suspension (1×106/mL) was put into a test tube, washed twice with PBS and centrifuged for 5 min at 1500 r/min. The supernate was discarded and the pallet was resuspended in 2 mL and 5 μmol/L DCFH-DA (2 mL PBS for the contrast groups), vortexed for 20 min in 37 °C water bath and centrifuged for 5 min at 1500 r/min. The supernate was discarded and the cells were resuspended in 600 μL PBS. The intensity of DCF green fluorescence was measured after DCFH-DA reaction with FACS. The wavelength of stimulation sub-laser within the FACS was 488 nm and the power was 10 mW. The results were expressed as mean fluorescence intensity (MFI).
DNA extraction was carried out according to the protocol of the reagent kit. (Promega, USA).
The sequences of primers are listed in Table 1. The total volume of PCR reaction was 50 μL, including 1 μL of each primer (20 pmol/μL), 5 μL of 10×PCR buffer, 5 μL of dNTPs (2 nmol/L), 0.4 μL of ExTaq DNA polymerase (5 U/μL) and 100 ng of extracted DNA sample. PCR reaction was carried on using PCR instrument (Perkin Elmer 2400, USA). The initial denaturation was at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 45 s, a denaturation at 56 °C for 45 s, an extension at 72 °C for 90 s. The final extension was at 72 °C for 7 min.
| Primers | Nucleotide sequence | |
| Upper | nt15791-15810 | 5'-ATCATTGGACAAGTAGCATC-3’ |
| Down | nt725-706 | 5'-GGTGAACTCACTGGAACGGG-3’ |
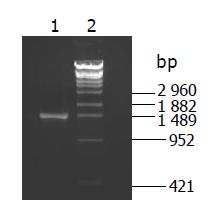
Two microliters of PCR product was loaded on 1.5% agrose gel for electrophoresis. If the mtDNA D-loop region was successfully amplified, a picture was taken (Figure 1) and the PCR product was purified with an instant PCR product purification kit (Promega, USA) and quantified with a spectrophotometer.
Sequencing reaction was completed with the sequencing kit of end termination by fluorescence labeled ddNTPs (ABI, USA). The total volume of sequencing PCR reaction was 10 μL, including 1 μL of sequencing primer, 6 μL of kit mixture, 3 μL of purified PCR product. Three sequencing primers (Table 2) were used to divide the replicated D-loop region into three overlapped segments. The reaction condition was as follows: the initial denaturation step was at 96 °C for 1 min, followed by 40 cycles of at 96 °C for 10 s and at 55 °C for 2 min. The sequencing reaction product was precipitated by 70% ethanol and loaded on a ABI Prism 310 sequencer (Perkin Elmer, USA).
| Primers | Nucleotide sequence | |
| Upper | nt111-nt130 | 5'-ACCCTATGTCGCAGTATCTG-3’ |
| Upper | nt16328-16347 | 5'-CGTACATAGCACATTACAGT-3’ |
| Down | nt16540-16514 | 5'-GTGGGCTATTTAGGCTTTATGACCCTG-3’ |
Taken the sequence of mtDNA D-loop from Cambridge sequence[9] as criterion, a comparison was made between the sequences of cancerous tissue and those of normal tissue. If the mtDNA D-loop sequence from cancerous tissue was different from normal tissue, the alteration was regarded as gene mutation.
Other data were expressed as mean±SD. Comparison between groups was carried out by t test. P<0.05 was considered statistically significant.
Eighteen gene mutations were found in the cancerous tissue from seven patients, among which four were microsatellite instabilities. Thus the mutation rate of mtDNA D-loop in the specimens of gastric cancer was 35% (Table 3).
| Location | Cambridge sequence | Cancer nucleotide | Normal tissue nucleotide |
| 16122 | T | C | T |
| 16221 | C | T | C |
| 16234 | C | Deletion | C |
| 16366 | T | C | T |
| 16401 | C | A | C |
| 16438 | G | A | G |
| 16465 | A | G | A |
| 16492 | A | Deletion | A |
| 16494 | C | T | C |
| 41 | C | T | C |
| 72 | T | C | T |
| 167 | C | G | C |
| 255 | G | A | G |
| 303 | (C)7 | (C)8 | (C)7 |
| 303 | (C)7 | (C)9 | (C)8 |
| 392 | T | C | T |
| 514 | (CA)5 | (CA)6 | (CA)5 |
| 567 | (C)6 | (C)7 | (C)6 |
Cell cycle and apoptosis could be detected by flow cytometry synchronously. In cell cycle, DNA was synthesized in synthesis (S) phase. As a result, the percentage of cells in synthesis phase could reflect cell proliferation. As shown in Table 4, level of ROS, rate of cell apoptosis and proliferation in mutation group were higher than those in control (P<0.05).
There was only 1 120 nt in the D-loop of mtDNA, but 18 mutations were detected in the 20 gastric cancer patients, indicating that the D-loop of mtDNA is a fragment with a high mutation rate.
Among the 18 gene mutations found in this work, four were microsatellite instabilities. Habano[10] researched the mitochondrial genome instability (mtGI) and nuclear micro-satellite instability (MSI ) in 62 gastric cancer tissues and found 10 mitochondrial PolyC instabilities (16%) and 7 MSI, among which 4 existed mtGI. Thus mtGI is correlated with nuclear MSI. Since nuclear MSI induces gene mutation in coding region, mtDNA mutation plays its role in the process of cancer genesis and progression by cooperating with the alteration of some nuclear genes.
Although a non-coding region, the mtDNA D-loop contains the initial site of heavy chain replication and the promoters for heavy and light chain transcription. Thus D-loop is responsible for the regulation of mtDNA replication and transcription, its mutation leads to mutations in coding region and change of protein synthesis, and finally affects the function of respiration chain which hampers the energy supply of cells and produces volume of ROS. ROS results in injury to the genome and then induces cancer.
High level of ROS is toxic through activating cell apoptosis and causing injuries to the genome. ROS might regulate cell apoptosis in the following ways. ROS is the message molecule of some transcription factors (such as Apaf-1) and can activate some useful components of cell apoptosis[11]. The increase of ROS is often accompanied with the decrease of intracellular anti-oxidant, resulting in imbalance between oxidant and reductive, which is just the common central step of cell apoptosis[2]. Most people believe that ROS is necessary for cell apoptosis. High level of ROS inspires cell necrosis or drives cells to the way from apoptosis to necrosis.
ROS not only participates in the process of cell apoptosis but also is a kinetin for cell division that promotes nuclear DNA mutation, cell mitosis and selective growth of tumor cells. ROS is relatively stable and easy to diffuse within cells and exist universally in various cell types. The formation and elimination of them are under strong cellular regulation. All the above properties make ROS extraordinary proper for second messengers. The level of intracellular ROS increases under extracellular stimulation signals such as cytokine and growth factor. Then they take part in cellular signal transduction. There exist two research hotspots at present as for the relation between ROS and cell proliferation. One is activation of MAP kinase family to promote cell mitosis, the other is activation of transcription factors such as NF-κB to facilitate gene expression.
In conclusion, the mutation of D-loop takes part in the carcinogenesis and progression of gastric cancer through the effect of increased ROS.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Copeland WC, Wachsman JT, Johnson FM, Penta JS. Mitochondrial DNA alterations in cancer. Cancer Invest. 2002;20:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Bianchi NO, Bianchi MS, Richard SM. Mitochondrial genome instability in human cancers. Mutat Res. 2001;488:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 325] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 618] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 5. | Habano W, Sugai T, Yoshida T, Nakamura S. Mitochondrial gene mutation, but not large-scale deletion, is a feature of colorectal carcinomas with mitochondrial microsatellite instability. Int J Cancer. 1999;83:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 609] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 7. | Lewis PD, Fradley SR, Griffiths AP, Baxter PW, Parry JM. Mitochondrial DNA mutations in the parotid gland of cigarette smokers and non-smokers. Mutat Res. 2002;518:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Cai L, Gao S, Yang Y, Zheng D, Wang W, Zhang G, Fuyong Q. Study on the relationship between apoptosis and reactive oxygen species of cancer cell lines induced by anticarcinogens. Zhonghua XueYeXue ZaZhi. 2001;22:249-251. [PubMed] |
| 9. | Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6507] [Cited by in RCA: 6452] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 10. | Habano W, Sugai T, Nakamura SI, Uesugi N, Yoshida T, Sasou S. Microsatellite instability and mutation of mitochondrial and nuclear DNA in gastric carcinoma. Gastroenterology. 2000;118:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Lovat PE, Ranalli M, Corazzari M, Raffaghello L, Pearson AD, Ponzoni M, Piacentini M, Melino G, Redfern CP. Mechanisms of free-radical induction in relation to fenretinide-induced apoptosis of neuroblastoma. J Cell Biochem. 2003;89:698-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |