Published online May 28, 2005. doi: 10.3748/wjg.v11.i20.3080
Revised: June 22, 2003
Accepted: October 12, 2003
Published online: May 28, 2005
AIM: NF-κB, regulate the expression of cytokine-inducible genes involving immune and inflammatory responses, will be potential therapy approach for allograft from rejection. In this study, we use pCMV-IκBαM vector to inhibit NF-κB activation and investigate the effect of pCMV-IκBαM in inhibition of T cells adhesion to endothelial cells.
METHODS: The NF-κB activity was detected with pNF-κB reporter gene and electrophoretic mobility shift assay. Expression of cell surface molecules was detected by RT-PCR and flow cytometer. The cell-cell adhesion assay was performed to determine the effect of pCMV-IκBαM in inhibition of T cells adhesion to endothelial cells.
RESULTS: We could find that NF-κB activity is inhibited by over-expression of non-degraded IκBα protein. Expression of adhesion molecules like ICAM-1, VCAM-1, and P-selectin as well as cell-cell adhesion were inhibited significantly by transfection of the pCMV-IκBαM vector.
CONCLUSION: Our results indicate that the pCMV-IκBαM, which inhibit the activity of NF-κB through over-expression of non-degraded IκBα protein, can be used for gene therapy in diseases involving NF-κB activation abnormally like organ transplantation via inhibiting cell adhesion.
- Citation: Wei JF, Sun K, Xu SG, Xie HY, Zheng SS. Inhibition of PMA-induced endothelial cell activation and adhesion by over-expression of domain negative IκBα protein. World J Gastroenterol 2005; 11(20): 3080-3084
- URL: https://www.wjgnet.com/1007-9327/full/v11/i20/3080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i20.3080
NF-κB, a DNA binding protein complex, is usually present in the cytosol as an inactive complex. IκBα, an associated protein, renders this complex inactive by shielding the nuclear localization signal (NLS). Upon IκB phosphorylation and its subsequent degradation, the NF-κB subunit P65 will translocate to the nucleus, where it binds to specific DNA sequences in the promoter region of several cytokine-inducible genes and up-regulates their transcription[1,2]. Extensive studies have showed that inhibition of NF-κB will be potential therapy approach for allograft from rejection[3-5]. The potent immunosuppressive agents FK506 and cyclosporin A (CsA) are reported to switch off gene transcription by inhibiting a key signaling phosphatase, calcinurin, which is involved in the activation of NF-κB[6,7]. Glucocorticoid, another major immunosuppressive agent, is also believed to work partly via inhibition of NF-κB activation[8]. The detail mechanism about inhibition of NF-κB protecting the graft is not clear till now.
Preview studies have shown that typical procedure of graft rejection is included in three steps: adhesion of alloantigen-activated leukocytes to the vascular endothelial cells, infiltration of alloantigen-activated leukocytes into the graft, activated leukocytes express immune factors which mediate graft tissue destruction[9]. The endothelial cell, as the first barrier faced the alloantigen, is thought to be the most important procedure during allograft rejection[10-13]. We suppose that NF-κB can be an important factor, which participate in the above three steps especially in the adhesion of leukocytes to the vascular endothelial cells.
In the present study, we focus on the transcriptional factor NF-κB in endothelial cells activation and adhesion to T cells. We use the vector encoding domain negative protein mutated at ser-32 and ser-36 IκBα to inhibit activation of NF-κB.
ECV304, an endothelial cell line, established from the vein of normal human umbilical cord, were obtained from Cell Bank of Chinese Academy of Science. Human Jurkat cell line was obtained from ATCC (American Type Cell Culture, USA) and cultured for adhesion assay. The following materials were used in this study: pCMV-IκBα, pCMV-IκBα M, pNF-κB Luc reporter vectors were obtained from Clontech (BD Biosciences, NJ, USA). Luciferase assay system, Gel shift assay system (Promega, WI, USA); MuLV reverase transcriptase, TRIzol, RPMI-1640 medium (Gibco, CA, USA); Geneticin (G418), FuGENE 6 transfection reagent, poly (dI-dC), Protease inhibitors cocktail (Roche, Monnehim, Germany); Protein assay kit (Bio-Rad, CA, USA). Phorbol myristate acetate (PMA) and all other reagents were purchased from Sigma (Sigma Chemical, USA).
All of the cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were maintained in a standard culture incubator with humidified air containing 50 mL/L CO2 at 37 °C.
We performed transfection according to the instruction manual of FuGENE 6 Transfection Reagent with modifications. Briefly, we seeded 1×105 cells in 1 mL RPMI 1640 containing 10% FBS medium in 24-well plate and incubated overnight until the ECV cells were 60% confluent. As for stable transfection, added 100 μL serum-free medium (SFM) containing 3 μL FuGENE 6 and 1 μg pCMV-IκBα or pCMV-IκBαM. The plate was incubated for 24 h for expression of reporter gene. Then we added 600 ng/mL G418 in every well and incubated for 7 d. After 7 d incubation, the concentration of G418 was decreased to 300 ng/mL and incubated for more than 3 wk. The over-expression of IκBα protein cells was selected using Western blotting. The cells stably transfected with pCMV-IκBα and pCMV-IκBαM were referenced to ECVWT and ECVMT, separately.
As for transient transfection, 100 μL SFM containing 3 μL FuGENE 6 and 1 μg pNF-κB Luc was added and cells were incubated for 24 h. After treated with PMA for 12 h, the cells were lysed and luciferase activity was measured using luciferase assay system.
Nuclear extracts were prepared as previously described[14]. Cytoplasmic buffer contained 10 mmol/L HEPES (pH 7.9), 0.1 mmol/L EDTA, 2 mmol/L MgCl2, 10 mmol/L KCl, 0.2% NP-40 and protease inhibitors cocktail solution was prepared to the cell pellet and incubated in ice for 10 min before centrifugation at 13000 g for 1 min. The pelleted nuclei were resuspended in nuclear buffer contained 20 mmol/L HEPES pH 7.9, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 150 mmol/L NaCl and protease inhibitors. The resuspended nuclei were incubated for 30 min on ice with vortexing interruptedly and centrifuged for 20 min at 13000 g.
The concentrations of samples were measured using protein assay kit with BSA protein standard solution as reference. Samples were stored at -70 °C until use.
The consensus oligonucleotide of NF-κB was obtained commercially from Santa Cruz and the sequence was shown as the following: NF-κB (5’-AGT TGA GGG GAC TTT CCC AGG C-3’). EMSA were performed according to Holmes’s protocol[15]. Briefly, NF-κB consensus oligonucleotide was radiolabeled with [γ-32P]ATP in the presence of T4 polynucleotide kinase. Five micrograms nuclear extract was incubated with labeled probe in 1 µg of poly(dI-dC) for 30 min. Complexes were separated on a 5% polyacrylamide gel in 0.5×TBE and dried using dryer (Bio-Rad, USA) and autoradiographed at -80 °C.
To study cytokine gene expression patterns, we used reverse transcription-polymerase chain reaction (RT-PCR), as previously described. Total RNA was extracted from ECV cells using TRIzol reagent according to the manufacturer’s recommendations. For cDNA synthesis, 4 µg total RNA was reverse transcribed with MuLV reverse transcriptase. Primer sequences and reaction conditions: Sequences of the primers used for RT-PCR analysis are described in Table 1. Amplifications were performed under the following conditions: 95 °C for 2 min, 94 °C for 45 s, 56 °C for 45 s, 72 °C for 45 s, totally 32 cycles. The final extension step was performed by one cycle at 72 °C for 10 min. Twenty-five microliters of reaction system was used including: 2 μL cDNA template, 1 μL sense primer, 1 μL anti-sense primer, 2 μL 25 mmoL MgCl2, 1 μL dNTP and 1.5 u Taq DNA polymerase. Reaction products were run by electrophoresis on a 1.5% agarose gel for 30-40 min at 100 V in 0.5×TBE buffer, and visualized with ethidium bromide staining under UV light. Relative expression level of ICAM-1 and VCAM-1 were defined as optical density ratio (Target gene/GAPDH) analyzed by Kodak digital science scanning system.
| Genes | Primers | Sequences | Amplifiers |
| ICAM-1 | Sense | 5'-CAGTGACCATCTACAGCTTTCCGG-3 | 555 bp |
| Anti-sense | 5'-GCTGCTACCACAGTGATGATGACAA-3’ | ||
| VCAM-1 | Sense | 5'-ACCCTCCCAAGGCACACACAG-3’ | 533 bp |
| Anti-sense | 5'-GTAAGTCTATCTCCAGCCTGTC-3’ | ||
| GAPDH | Sense | 5'-ATGGCACCGTCAAGGCTGAG-3’ | 225 bp |
| Anti-sense | 5'-GCAGTGATGGCATGGACTGT-3’ |
ECVMT and ECVWT cells were treated with PMA (50 nmol/L) for 12 h. Then cells were harvested and washed thrice using cold PBS. Then the cells were incubated with FITC-labeled CD54(ICAM-1), FITC-labeled CD62 (VCAM-1) or PE-labeled CD106(P-selectin) antibodies. The fluorescence densities were determined using flow cytometer (Coulter, USA) and the experiment was repeated thrice.
We used Jurkat cells for lymphocyte-endothelial cell adhesion assay. The adhesion procedure was performed according to Roy[16]. Briefly, monolayers of cells were seeded at a density of 104 cells/well in 96-well plate (Becton Dickinson, NJ, USA). After 24 h of seeding, the cells were treated with PMA (50 nmol/L) for 12 h. Before cell-cell adhesion assay, the ECV monolayers were washed thrice with PBS. Jurkat T-cells (2×105 cells/well) were co-cultured with ECV monolayer for 2 h in a culture incubator. After the co-culture period, the non-adherent Jurkat T-cells were removed by washing each well four times with PBS carefully. Jurkat cell adhesion was determined by visual counting under a phase-contrast microscope. Both Jurkat cells and ECV were counted in five fields of each well at 100× magnification by two individuals, and the average values were taken. The adhesion rate is expressed as the number of attached Jurkat cells per 100 ECV cells.
Results are presented as mean±SD of at least three separate experiments. Differences between means of groups were determined by Student’s t-test. The level of significance was set at P<0.05 or P<0.01.
We stably transfected the ECV304 cells with the pCMV-IκBαM vector, which encoded domain negative IκBα protein mutated at ser-32 and ser-36 and also with the pCMV-IκBα as control. Firstly, we extracted the total protein after being treated with PMA (50 nmol/L) for 45 min. The expression level of IκBα was determined with specific antobody. As shown in Figure 1A, IκBα level was higher in ECVMT cells after treated with PMA compared with ECVWT cells. To determine the NF-κB activity further, the nuclear proteins were extracted after treated with PMA for 45 min. These proteins were applied to EMSA. Figure 1B showed us that pCMV-IκBαM could inhibit the activity of NF-κB significantly.
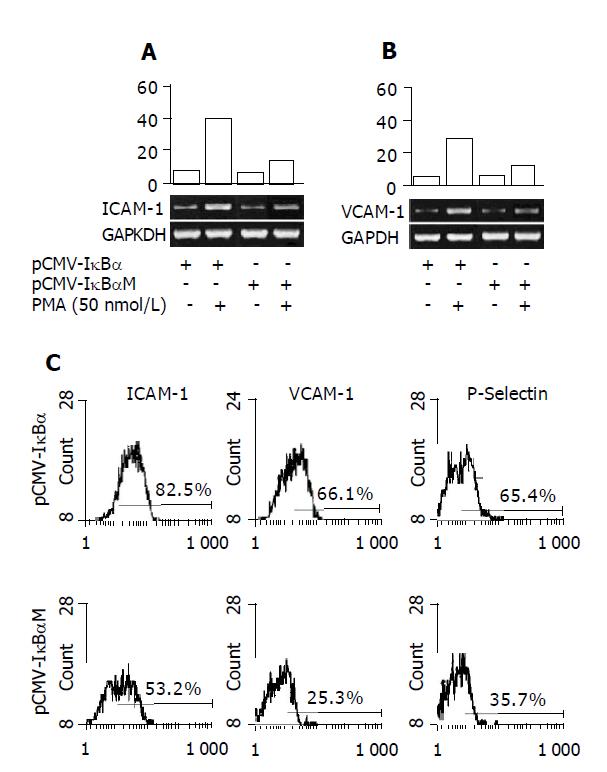
Adhesion molecules expression in endothelial cells is dependent, at least in part, on the activation of NF-κB[1]. To determine whether the pCMV-IκBαM can inhibit the expression of adhesion molecules, we performed RT-PCR to determine the mRNA level of adhesion molecules in the total RNA extracted from the cells treated with PMA (50 nmol/L) for 12 h. The results were shown as ratio compared to housekeeping gene GAPDH expression. As shown in Figures 2A and 2B, we can see that the ICAM-1 and VCAM-1 mRNA levels are inhibited significantly in ECVMT cells compared to ECVWT cells (40.3% vs 18.7% for ICAM-1 and 27.3% vs 16.7% for VCAM-1). We also compare the protein levels of various adhesion molecules by flow cytometer. The cells were harvested after stimulation with PMA (50 nmol/L) for 12 h. As shown in Figure 2C, the pCMV-IκBαM can inhibit the expression of adhesion molecules including ICAM-1, VCAM-1 and P-selectin. As for ICAM-1, 82.5% cells expressed ICAM-1 in ECVWT cells after being treated with PMA (50 nmol/L) compared 53.2% positive cells in ECVMT cells. Almost the same results were found in VCAM-1 and P-selectin expression between ECVMT cells and ECVWT cells (25.3% vs 66.1% for VCAM-1; 65.4% vs 35.7% for P-selectin).
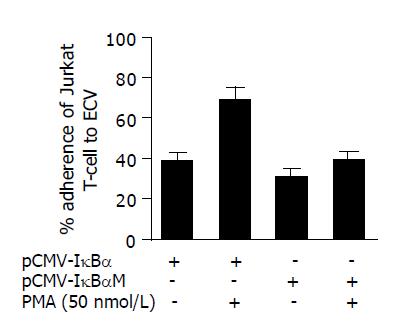
We then evaluated the effect of pCMV-IκBαM on the adhesion of human T lymphocyte to ECV304 cells as mentioned in Methods section. After the ECV cells were treated with PMA (50 nmol/L) for 24 h, the Jurkat T cells were added and co-incubated for further 2 h. Then we calculated the adherent cells using phase-contrast microscope after non-adherent cells were washed away using cold PBS (Figure 3). We found that the pCMV-IκBαM could inhibit the adhesion of Jurkat T cells to ECV cells from 71.4±5.2% to 42.2±3.7% after being treated with PMA for 12 h (P<0.05).
The adhesion of leukocyte to the vascular endothelial cells is a critical step in the immunological response and involves recruitment and infiltration of leukocytes to the site of tissue injury or allograft. The endothelial cell, as the first barrier faced the alloantigen, will be activated upon stimulation like cytokines (e.g., PMA, IL-1, TNF-α,) in vitro as well as in vivo at sites of allograft. Activated endothelial cells express adhesion molecules to assist adhesion between activated T cells and endothelial cells[18,19]. Among these molecules are P- and E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), on the endothelial cells, and their respective counter receptors, P-selectin glycoprotein ligand-1 (PSGL-1), leukocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4), on the leukocytes[17]. Activated endothelial cells also can secrete chemokines such as IL-6, IL-8 and MCP-1[20,21], which can recruit leukocyte. ICAM-1, which has been studied extensively, binds to its ligand LFA-1 on lymphocytes and promotes lymphocytes binding to the endothelium and facilitates the lymphocytes to invade the graft[22-24]. Blockade of ICAM-1, either with monoclonal antibodies or with antisense oligodeoxynucleotides, has been shown to decrease reperfusion injury and prolong the survival of allograft[25-27].
Activation of endothelial cells requires multiple transcriptional factors. Nuclear factor κB, one of such transcription factors, is held in the cytoplasm by inhibitory IκB proteins (IκBs) and regulates many genes involving immune and inflammatory pathways such as various proinflammatory cytokines, adhesion molecules and apoptosis-associated factors[8,28]. Accumulating evidence clearly demonstrates that blocking the activity of NF-κB might arrest the progression of acute rejection by interrupting the activation of genes of major inflammatory cytokines and adhesion molecules[29]. We use the pCMV-IκBαM vector, which encodes domain negative nondegraded IκBα protein mutated at ser-32 and ser-36 to inhibit the activation of NF-κB. We found that the adhesion molecules including ICAM-1, VACM-1 and P-selectin were down-regulated under inhibition of NF-κB. We also mimic the procedure of cell-cell adhesion in vitro between endothelial cells and T cells. The results told us that inhibition of NF-κB could inhibit endothelial cell activation and decrease cell adhesion.
In conclusion, we propose that pCMV-IκBαM vector can be used as novel immunological strategy. Further explorations were needed to verify the effect of pCMV-IκBαM to protect the allograft from rejection in vivo.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2972] [Cited by in RCA: 3213] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 2. | Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Bonham CA, Peng L, Liang X, Chen Z, Wang L, Ma L, Hackstein H, Robbins PD, Thomson AW, Fung JJ. Marked prolongation of cardiac allograft survival by dendritic cells genetically engineered with NF-kappa B oligodeoxyribonucleotide decoys and adenoviral vectors encoding CTLA4-Ig. J Immunol. 2002;169:3382-3391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Finn PW, Stone JR, Boothby MR, Perkins DL. Inhibition of NF-kappaB-dependent T cell activation abrogates acute allograft rejection. J Immunol. 2001;167:5994-6001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Suzuki J, Morishita R, Amano J, Kaneda Y, Isobe M. Decoy against nuclear factor-kappa B attenuates myocardial cell infiltration and arterial neointimal formation in murine cardiac allografts. Gene Ther. 2000;7:1847-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Kaibori M, Sakitani K, Oda M, Kamiyama Y, Masu Y, Nishizawa M, Ito S, Okumura T. Immunosuppressant FK506 inhibits inducible nitric oxide synthase gene expression at a step of NF-kappaB activation in rat hepatocytes. J Hepatol. 1999;30:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Frantz B, Nordby EC, Bren G, Steffan N, Paya CV, Kincaid RL, Tocci MJ, O'Keefe SJ, O'Neill EA. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994;13:861-870. [PubMed] |
| 8. | Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 459] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | el-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol. 2002;14:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Briscoe DM, Alexander SI, Lichtman AH. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr Opin Immunol. 1998;10:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Denton MD, Davis SF, Baum MA, Melter M, Reinders ME, Exeni A, Samsonov DV, Fang J, Ganz P, Briscoe DM. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr Transplant. 2000;4:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Paul LC, Baldwin WM, van Es LA. Vascular endothelial alloantigens in renal transplantation. Transplantation. 1985;40:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Rose ML. Endothelial cells as antigen-presenting cells: role in human transplant rejection. Cell Mol Life Sci. 1998;54:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, Benoit V, Merville MP. Nuclear factor-kappa B, cancer, and apoptosis. Biochem Pharmacol. 2000;60:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Holmes-McNary M, Baldwin AS. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477-3483. [PubMed] |
| 16. | Roy S, Sen CK, Kobuchi H, Packer L. Antioxidant regulation of phorbol ester-induced adhesion of human Jurkat T-cells to endothelial cells. Free Radic Biol Med. 1998;25:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Dedrick RL, Bodary S, Garovoy MR. Adhesion molecules as therapeutic targets for autoimmune diseases and transplant rejection. Expert Opin Biol Ther. 2003;3:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Mozaffarian N, Casadevall A, Berman JW. Inhibition of human endothelial cell chemokine production by the opportunistic fungal pathogen Cryptococcus neoformans. J Immunol. 2000;165:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kokura S, Yoshida N, Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic Biol Med. 2002;33:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol Cell Physiol. 2003;285:C1036-C1046. [PubMed] |
| 21. | Anrather J, Csizmadia V, Brostjan C, Soares MP, Bach FH, Winkler H. Inhibition of bovine endothelial cell activation in vitro by regulated expression of a transdominant inhibitor of NF-kappa B. J Clin Invest. 1997;99:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4705] [Cited by in RCA: 4728] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 23. | Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 904] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 24. | Borthwick NJ, Akbar AA, Buckley C, Pilling D, Salmon M, Jewell AP, Yong KL. Transendothelial migration confers a survival advantage to activated T lymphocytes: role of LFA-1/ICAM-1 interactions. Clin Exp Immunol. 2003;134:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Poston RS, Ennen M, Pollard J, Hoyt EG, Billingham ME, Robbins RC. Ex vivo gene therapy prevents chronic graft vascular disease in cardiac allografts. J Thorac Cardiovasc Surg. 1998;116:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Feeley BT, Park AK, Alexopoulos S, Hoyt EG, Ennen MP, Poston RS, Robbins RC. Pressure delivery of AS-ICAM-1 ODN with LFA-1 mAb reduces reperfusion injury in cardiac allografts. Ann Thorac Surg. 1999;68:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Stepkowski SM. Application of antisense oligodeoxynucleotides for organ transplantation. Transplant Proc. 1998;30:2142-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3641] [Article Influence: 117.5] [Reference Citation Analysis (1)] |