Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.303
Revised: April 8, 2004
Accepted: May 25, 2004
Published online: January 14, 2005
AIM: Aloe vera, plant extracts of Aloe barbadensis miller, is widely used in phytomedicine. The first case of acute hepatitis due to this compound was described.
METHODS: Description of a clinical case.
RESULTS: Hepatitis in a 57-year old female could be linked to the ingestion of Aloe barbadensis miller compounds. The patient´s hepatitis resolved completely after discontinuing this medication.
CONCLUSION: The case emphasizes the importance of considering phytopharmaceutical over-the-counter drugs as causative agents of hepatitis.
- Citation: Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis induced by an Aloe vera preparation: A case report. World J Gastroenterol 2005; 11(2): 303-304
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.303
Aloe vera is a chemically ill-defined extract of the Aloe barbadensis miller plant. There is no doubt that this compound is bioactive[1]. Phytomedicine ascribes anti-inflammatory, analgetic, liver-protective, anti-proliferative, anti-carcinogenic, anti-aging, and laxative effects to this plant[2-5]. These effects are thought to be the result of radical scavenging, inhibition of COX-2, and immuno-modulatory mechanisms. The drug is widely used as a self-prescribed anti-aging drug in Western European countries as well as in the USA. Even though it is in widespread use as an over-the-counter drug, its toxicology has not been systematically examined. Here, we describe the first case of liver damage associated with Aloe vera ingestion.
A 57-year old female patient presented to our department with an 1 wk history of progressive jaundice, pruritus, acholic bowel movements, and right-upper quadrant abdominal discomfort. Past medical history did not reveal any preexisting liver disease. There was no history of illicit drug use and no sexual promiscuity. In the hope to delay aging, the patient had begun using Aloe vera tablets containing 500 mg of an extract of Aloe barbadensis miller about 4 wk before admission. She also used zinc and vitamin C supplements as directed by the manufacturers. The Aloe vera tablets had been purchased in Spain as they were less expensive there than in Germany.
Clinical examination revealed a mildly overweight (70 kg bodyweight, 167 cm height) jaundiced patient with right-upper quadrant discomfort on deep palpation. Liver and spleen sizes were normal. There was no lymphadenopathy. The remainder of the physical examination was unremarkable.
On abdominal ultrasound examination, a reduced echogenicity of a normal sized liver was noted. Dilatation of intra- or extrahepatic bile ducts was absent. Patency of the hepatic artery, portal vein, and hepatic veins was ascertained using Doppler ultrasound. Splenic size was normal, the examination of kidneys, pancreas and retroperitoneal space was normal as well.
Laboratory abnormalities included a bilirubin concentration of 8.9 mg/dL (normal: <1.1mg/dL), ALAT 1480 U/L (normal: <22 U/L), ASAT 711 U/L (normal: <15 U/L), LDH 506 U/L (normal<240 U/L), alkaline phosphatase 265 U/L (normal: <160 U/L), GGTP 244 U/L (normal: <18 U/L). Creatinine, serum electrolytes, amylase, total protein, electrophoresis, serum concentrations of IgG, IgA, and IgM, white blood cell count, hemoglobin concentration, platelet count, and differential blood cell count were all within the normal range. Coeruloplasmin concentration was normal as was the alpha-1-antitrypsin concentration.
Serologic examinations for hepatitis A, C, and E infection were negative. HCV-PCR was negative. Anti-HBc-IgG and anti-HBs-IgG were positive, while HBsAg and anti-HBc-IgM were negative. There was no serologic evidence for recent infections with cytomegalovirus, Epstein-Barr-virus, or herpes virus. Autoimmune markers showed negative titers for antimitochondrial and borderline titers for antinuclear antibodies (1:40; normal titer defined as <1:40).
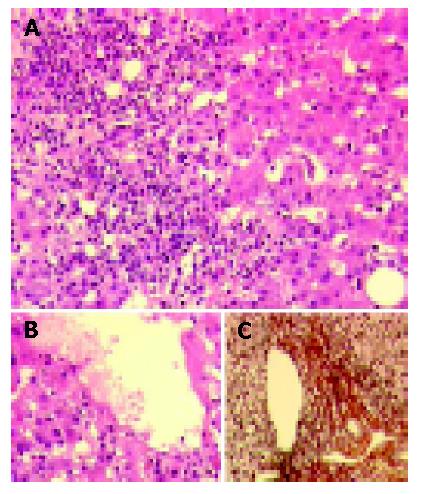
Liver biopsy was performed and revealed severe acute hepatitis with portal and acinar infiltrates predominantly consisting of lymphocytes, plasma cells, and eosinophilic granulocytes along with bridging necrosis and bilirubinostasis (Figure 1).
All medications were withheld and aminotransferases as well as the bilirubin concentration gradually returned to normal levels over the course of several months. Two weeks after admission, ALAT concentration was 226 U/L, 5 mo after discharge ALAT concentration was 180 U/L, and 1 year after discharge ALAT concentration decreased to 40 U/L. The patient became completely asymptomatic within a week and has remained so ever since.
To our knowledge, this is the first case of toxic hepatitis that can be ascribed to an Aloe vera preparation. The Aloe vera preparation was the only active compound ingested during the period preceding the occurrence of toxic hepatitis. This, the exclusion of common alternative diagnoses, and the rapid improvement and resolution of liver damage following discontinuation of Aloe vera provide strong evidence that the Aloe vera preparation has caused the acute hepatitis.
It is very unlikely that the ongoing concomitant medication with vitamin C and zinc supplements may have been involved, as these substances are not allergenic and hepatotoxic.
Aloe vera, the dried extract from the leaves of Aloe barbadenis miller plants, contains several alkaloids that may induce or block hepatic enzyme systems such as cytochrome P450 as well as the enzymes of ethanol metabolism[6]. This interference with detoxification processes leading to dose-related liver damage or direct cytotoxic effects of Aloe[7] or biotransformed constituents[8] are probably not important mechanisms in our case as the resolution of liver damage occurred much too slowly. It is more likely that an idiosyncratic immunological mechanism (hypersensitivity) is responsible for the hepatitis. A role for hypersensitivity is further supported by the presence of eosinophilic granulocytes in the periportal fields seen in the liver biopsy. As there was no evidence for the presence of an autoimmune hepatitis, especially no hypergammaglobinemia or markedly elevated autoantibody titers, we propose that this liver damage was triggered by the Aloe vera preparation. Hypersensitivity to Aloe - which may have a delayed presentation - has been described in humans[9]. Several compounds present in Aloe vera may interact with the host’s immune system[10]. This activation of the immune system was also discussed as a possible mechanism for a reported anti-tumor activity of Aloe vera[11]. The interactions with the immune system may inhibit the release or cause the rapid detoxification of reactive oxygen species[12]. This antioxidant effect of Aloe vera is also implicated in the potential anti-hepatocarcinogenic and hepatoprotective properties of the drug[13-17]. Conversely, some constituents of Aloe vera have been reported to be biotransformed to mutagenic compounds with equivocal evidence for in vivo carcinogenicity. The growth-inhibiting effect of Aloe vera is mediated through pro-apoptotic pathways. One could speculate that this effect may also be present in normal liver cells and leads to liver damage or to the triggering of an immune response directed toward intracellular antigens. A similar mechanism may be present in kidney damage associated with other Aloe species.
Herbal medicines are widely used in almost all segments of the population. A variety of herbal medicines can cause liver damage. Again, our case emphasizes that phytotherapeutic drugs should be subjected to the same toxicologic studies and pharmacovigilance that synthetic drugs are subjected to.
We thank Mrs. U. Moser, University of Bonn, for online reference acquisition.
Edited by Wang XL
| 1. | Logarto Parra A, Silva Yhebra R, Guerra Sardiñas I, Iglesias Buela L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine. 2001;8:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Swanson LN. Therapeutic value of Aloe Vera. US Pharmacist. 1995;26-35. |
| 3. | Fischer JM. Medical use of aloe products. US Pharmacist. 1982;7:37-45. |
| 4. | Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo-controlled, double-blind study. Trop Med Int Health. 1996;1:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Ikeno Y, Hubbard GB, Lee S, Yu BP, Herlihy JT. The influence of long-term Aloe vera ingestion on age-related disease in male Fischer 344 rats. Phytother Res. 2002;16:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chung JH, Cheong JC, Lee JY, Roh HK, Cha YN. Acceleration of the alcohol oxidation rate in rats with aloin, a quinone derivative of Aloe. Biochem Pharmacol. 1996;52:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Avila H, Rivero J, Herrera F, Fraile G. Cytotoxicity of a low molecular weight fraction from Aloe vera (Aloe barbadensis Miller) gel. Toxicon. 1997;35:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Mueller SO, Stopper H, Dekant W. Biotransformation of the anthraquinones emodin and chrysophanol by cytochrome P450 enzymes. Bioactivation to genotoxic metabolites. Drug Metab Dispos. 1998;26:540-546. [PubMed] |
| 9. | Morrow DM, Rapaport MJ, Strick RA. Hypersensitivity to aloe. Arch Dermatol. 1980;116:1064-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Hart LA, van Enckevort PH, van Dijk H, Zaat R, de Silva KT, Labadie RP. Two functionally and chemically distinct immunomodulatory compounds in the gel of Aloe vera. J Ethnopharmacol. 1988;23:61-71. [PubMed] [DOI] [Full Text] |
| 11. | Corsi MM, Bertelli AA, Gaja G, Fulgenzi A, Ferrero ME. The therapeutic potential of Aloe Vera in tumor-bearing rats. Int J Tissue React. 1998;20:115-118. [PubMed] |
| 12. | Singh RP, Dhanalakshmi S, Rao AR. Chemomodulatory action of Aloe vera on the profiles of enzymes associated with carcinogen metabolism and antioxidant status regulation in mice. Phytomedicine. 2000;7:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Shamaan NA, Kadir KA, Rahmat A, Ngah WZ. Vitamin C and aloe vera supplementation protects from chemical hepatocarcinogenesis in the rat. Nutrition. 1988;14:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kim HS, Kacew S, Lee BM. In vitro chemopreventive effects of plant polysaccharides (Aloe barbadensis miller, Lentinus edodes, Ganoderma lucidum and Coriolus versicolor). Carcinogenesis. 1999;20:1637-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, De Castri D, Moscheni C, Annoni G. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000;87:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Yagi A, Kabash A, Okamura N, Haraguchi H, Moustafa SM, Khalifa TI. Antioxidant, free radical scavenging and anti-inflammatory effects of aloesin derivatives in Aloe vera. Planta Med. 2002;68:957-960. |
| 17. | Yagi A, Kabash A, Mizuno K, Moustafa SM, Khalifa TI, Tsuji H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from aloe vera gel. Planta Med. 2003;69:269-271. |