Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.268
Revised: February 8, 2004
Accepted: March 18, 2004
Published online: January 14, 2005
AIM: Hepatic fibrogenesis has close relation with hepatic stellate cells (HSC) and tissue inhibitors of metalloproteinase (TIMP). Oxymatrine (OM) is a kind of Chinese herb that is found to have some effects on liver fibrosis. We aimed to determine the effects of OM on hepatic fibrosis and explore the possible mechanism.
METHODS: Thirty-two rats were randomly divided into four groups; 16 were used to develop hepatic fibrosis by carbon tetrachloride (CCl4) and treated with or without OM, and 16 were used as controls. The expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and α-smooth muscle actin (α-SMA) in the livers of rats was detected by immunohisto-chemical assay. Liver pathology was determined by H&E staining and reticulum staining.
RESULTS: In CCl4-injured rats, the normal structure of lobules was destroyed, and pseudolobules were formed. Hyperplasia of fibers was observed surrounding the lobules. While the degree of fibrogenesis in liver tissues was significantly decreased in those rats with OM-treatment compared with those without OM treatment. The pseudolobules were surrounded by strong, multi-layer reticular fibers, which netted into pseudolobules in CCl4-injured rats, however, there was a significant decrease in reticular fibers in OM-treated rats. The expression of TIMP-1 in hepatic cells was weak in control groups, but strong in CCl4-injured groups, however, the expression of TIMP-1 was significantly inhibited by OM (F = 52.93, P<0.05). There was no significant change in the expression of α-SMA between CCl4-injured rats with or without OM treatment (F = 8.99, P>0.05).
CONCLUSION: OM effectively inhibits CCl4-induced fibrogenesis in rat liver tissues, probably by reducing the expression level of TIMP-1.
-
Citation: Shi GF, Li Q. Effects of oxymatrine on experimental hepatic fibrosis and its mechanism
in vivo . World J Gastroenterol 2005; 11(2): 268-271 - URL: https://www.wjgnet.com/1007-9327/full/v11/i2/268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.268
Significant progress in the cellular and molecular mechanisms of hepatic fibrogenesis has been achieved over the last decades[1-5]. Hepatic fibrogenesis is known to be a gradual and dynamic process, which involves a series of complicated changes in cells, cytokines and extra cellular matrix (ECM). Especially, it has close relation with the hepatic stellate cells (HSC)[6] and tissue inhibitors of metalloproteinase (TIMP)[7].
Oxymatrine (OM) is a kind of Chinese herb which can inhibit hepatitis B and C viruses[8-12]. It was originally used for the treatment of chronic viral hepatitis. Later, it was found to have some effects on liver fibrosis[13]. However, there is little evidence of the underlying mechanism. In this study, we determined the effects of OM on experimental hepatic fibrosis, and explored the possible mechanism in vivo.
Thirty-two adult male Sprague Dawley rats, weighing 190-230 g (provided by Experimental Animal Center of Chinese Academy, Shanghai, China) were included in the experiments. A rat model of chronic hepatic injury was produced by carbon tetrachloride (CCl4) IH (hypodermic injection) in 16 rats, and 16 rats were used as controls.
The rats were randomly divided into four groups. In group 1 (CCl4 group, n = 8), CCl4 (0.3 mL/100 g weight, Shanghai Lingfeng Chemicals Reagent Company, Shanghai, China) was injected at the back hypodermically twice per wk for 10 wk to develop a model of hepatic fibrosis. In group 2 (CCl4+OM group, n = 8), CCl4 was given as in group 1 together with OM (60 mg/kg weight, Shanghai Chemicals Reagent Company, China Pharmaceutical Corporation, Shanghai, China), once daily for 10 wk. In group 3 (OM control group, n = 8), OM was given as in group 2 but without CCl4. In group 4 (blank control group, n = 8), olive oil (0.3 mL/100 g weight, Ningxia Pharmaceutical Factory, Ningxia, China), instead of CCl4, was injected at the back hypodermically. After 10-wk injection, all animals were sacrificed under narcosis, and their right liver lobes were immediately excised. The specimens were fixed in formaldehyde for the preparation of serial paraffin sections at 5-μm thickness.
Liver sections were stained with hematoxylin and eosin (H and E) for the assessment of liver cell degeneration and necrosis, infiltration of inflammatory cells, hyperplasia of the lattice fibers and formation of collagen bundles and pseudolobules. Other sections were stained with Von Gieson and Masson special staining for the assessment of fiber structure of the livers and analysis of hyperplasia of liver fiber tissues by computer image analysis system.
TIMP-1 expression was determined by the immunohistochemical ABC method. The serial paraffin sections of liver samples were deparaffinized and rehydrated after being immersed in 0.3% peroxide-ethanol solution at room temperature for 10 min, and digested with 0.1% pancreatic protease at 37 °C for 60 min. Rabbit anti-TIMP-1 antibody (working concentration 1:100, Santa Cruz Biotechnology Company, LA, USA) was added at 37 °C for 60 min, then the samples were put at 4 °C overnight. Then biotinylated goat anti-rabbit antibody was added at 37 °C for 60 min, and ABC compound (working concentration 1:100, Huamei Company, Shanghai, China) was incubated at 37 °C for 45 min. Finally, diaminobenzidine tetrahydrochloride (DAB, Huamei Company) was added to develop color for microscopic examination and photography.
For immunohistochemical staining of α-SMA, the paraffin sections were deparaffinized and rehydrated after being immersed in 0.3% peroxide-ethanol solution at room temperature for 10 min and digested with microwave for 10 min. After incubation with the rat anti-human α-SMA monoclonal antibody (working concentration 1:100, Long-island Antibody Diagnosis Company, Shanghai, China) at 37 °C for 60 min and at 4 °C overnight, the sections were incubated with the rabbit anti-rat antibody (working concentration 1:100, Long-island Antibody Diagnosis Company) at 37 °C for 60 min, and finally with DAB to develop color for microscopic examination and photography.
χ2-test was performed with SPSS 10.0.
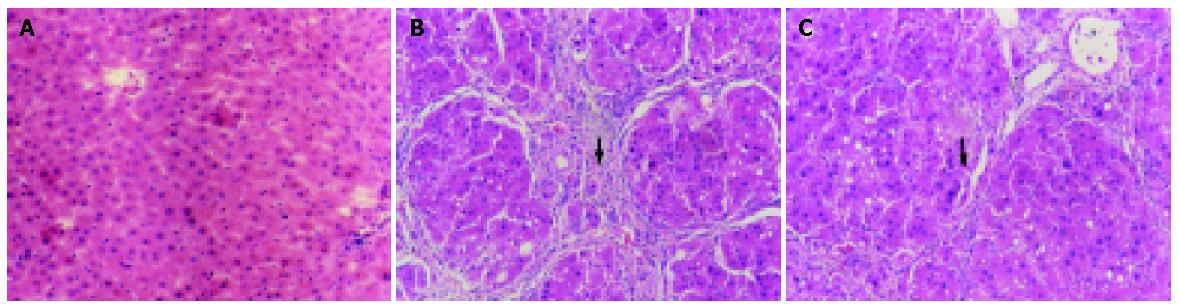
In the two control groups (OM control group and blank control group), the structure of liver lobules was normal, central vein was clear and the size and shape of hepatic cells were normal and distributed centrifugally (Figure 1A). In the CCl4 group, the normal structure of lobules was destroyed, and pseudolobules were formed. Liver cells swelled, with lipid vacuoles in cytoplasm. Hyperplasia of fibers was observed surrounding the lobules. The hepatic lobules were encysted and separated by collagen bundles, and associated with bile duct “proliferation”, infiltration of inflammatory cells, bordering of the portal areas. Abundant fiber tissues were present, extended outwards in stellar shape. Cholestasis was seen in some bile duct cells. Hyperplasia of ovum shape cells was observed in collagen bundles. Mitotic figures were seen in particular individual cells (Figure 1B). In CCl4+OM group, the normal structure of lobules was also destroyed, with pseudolobule formation. Liver cells swelled and degenerated, with lipid vacuoles in cytoplasm. The hepatic lobules were encysted and separated by collagen bundles, with infiltration of inflammatory cells. However, these changes were significantly milder compared with CCl4 group (Figure 1C).
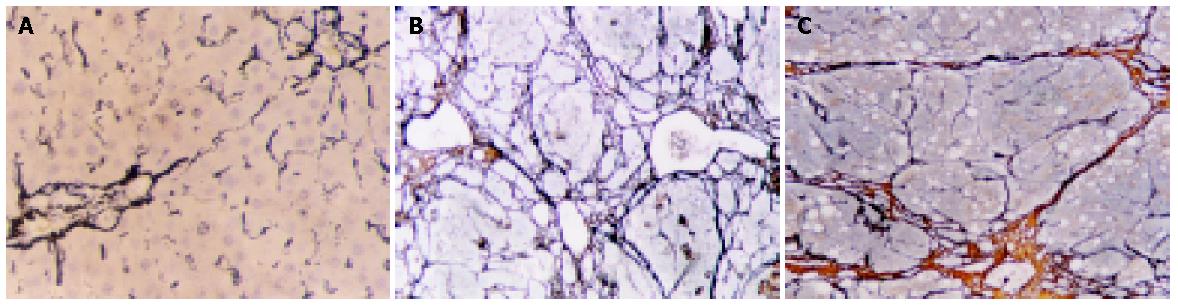
In the two control groups, there were only a few fiber tissues seen in portal areas. There was no significant difference between OM control group and blank control group (Figure 2A). However, in CCl4 group, the pseudolobules were surrounded by strong, multi-layer reticular fibers, which netted into pseudolobules (Figure 2B). In CCl4+OM group, the pseudolobules were surrounded by multi-layer reticular fibers, which netted into pseudolobules. However, there was a significant decrease in reticular fibers in this group, compared with CCl4 group (Figure 2C).
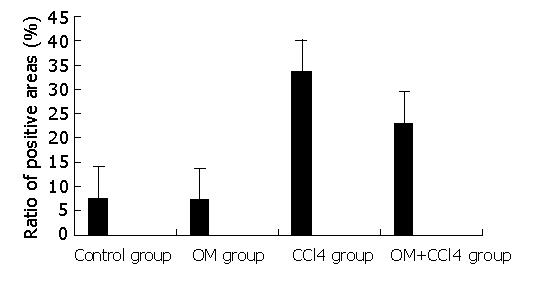
Image pattern analysis showed that the expression of collagen in the experimental groups (CCl4 group and CCl4+OM group) was much stronger than that in the two control groups. Moreover, the expression of collagen in CCl4 group was significantly stronger than that in CCl4+OM group (F = 31.89, P<0.05) (Figure 3).
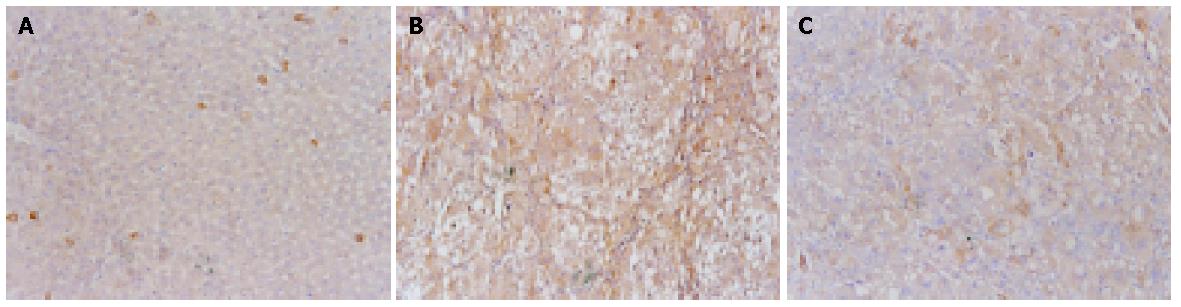
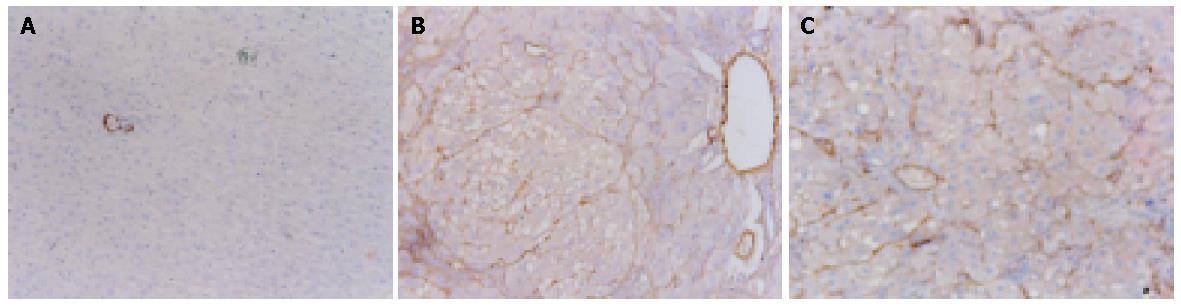
Cytoplasmic expression of TIMP-1 antigen was detected in most hepatic cells in the experimental rats, but only in a few individual liver cells and hepatic stellate cells (HSCs) in the control rats (Figure 4A). The expression of TIMP-1 in hepatic cells was weak in control groups, but strong in experimental groups (Figure 4B). There was a significant decline in the expression of TIMP-1 in CCl4+OM group, compared with that in CCl4 group (F = 52.93, P<0.05) (Figure 4C, Table 1).
| Groups | n | TIMP-1 | a-SMA |
| CCl4 group | 8 | 35.07±6.50 | 24.89±5.32 |
| CCl4+OM group | 8 | 27.01±5.88a | 21.64±2.36 |
| OM group | 8 | 2.16±1.16 | 4.46±1.47 |
| Control group | 8 | 2.48±1.40 | 12.52±17.6 |
α-SMA antigen in control rat liver was detected in myofibroblasts and vascular endothelial cells. The expression of α-SMA in experimental groups was significantly stronger than that in control groups (Figure 5A). There was no significant difference between CCl4 group and CCl4+OM group (F = 8.99, P>0.05) (Figure 5B, C, Table 1).
Studies on the pathogenesis of hepatic fibrosis have shown that HSCs within the space of Disse experience a transform, in the wake of liver injures by various causes. In normal liver, HSCs store vitamin A and show minimal proliferation and collagen synthesis. However, in an injured liver, HSCs lose vitamin A and transform into myofibroblasts (MFB), called activated HSCs, which express α-SMA and have the function of contractibility, proliferation and fibrogenesis[12]. HSCs synthesize a number of collagens, and enzymes that inhibit degeneration of extracellular matrix (ECM), and some cytokines that promote fibrosis. Thus, the balance between the deposition and degeneration of ECM is broken, leading to the startup and development of hepatic fibrosis. Some studies have shown that TIMP is a very important promoting factor of hepatic fibrosis, and it inhibits matrix metal protease (MMP) to deposit ECM[13]. Strong expression of TIMP-1 reflects the severity of hepatic fibrosis. On the other hand, OM is a kind of alkaloid, extracted from a Chinese herb, Guangdou Root. Some reports have shown that OM inhibits the secretion of IL-1, IL-6 and TNF-α from Kupffer cells, the proliferation of fibroblasts and expression of PIIIP mRNA[11]. To our knowledge, there are no reports on the effects of OM on activation of HSCs and expression of TIMP-1 in liver.
In our present study, the pathologic observation and reticular collagen staining demonstrated that the expression of collagen in CCl4+OM group was significantly lower than that in CCl4 group. Our results indicate that OM has a potent interference effect on hepatic fibrosis. In addition, the results of immunohistochemical staining showed that the expression of TIMP-1 in CCl4+OM group was significantly lower than that in CCl4 group. Combining the above quantitative data of reticular collagen, we noticed that the stronger the expression of TIMP-1 was the more reticular collagens were secreted, and vice versa. We suggest that TIMP-1 plays an important role in the process of hepatic fibrogenesis. One possible mechanism of OM’s anti-fibrotic effect lies in its inhibition of expression of TIMP[14]. Several reports have shown that the expression levels of TIMP-1 reflect the severity of alcohol-induced hepatic fibrosis. Accordingly, there seems to be some correlation between the expression levels of TIMP-1 and the severity of hepatic fibrosis.
The present study showed that the expression of α-SMA in the liver in experimental groups was significantly stronger than that in control groups. However, there was no significant difference between CCl4 group and CCl4+OM group. It is presumed that OM does not have a direct effect on the activation of HSCs, whereas it influences the function of myofibroblasts[15]. Nevertheless, OM inhibits the secretion of collagen fibers and expression of TIMP-1, which in turn have interference effect on hepatic fibrosis.
In conclusion, OM has an interference effect on hepatic fibrosis, and one possible mechanism lies in its inhibitory effect on the expression of TIMP-1 in the liver.
The authors thank Mr. Gang Qin (Huashan Hospital) for the preparation of the manuscript.
Edited by Xia HHX Proofread by Zhu LH
| 1. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 2. | Dodig M, Mullen KD. New mechanism of selective killing of activated hepatic stellate cells. Hepatology. 2003;38:1051-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Cai Y, Shen XZ, Wang JY. Effects of glycyrrhizin on genes expression during the process of liver fibrosis. Zhonghua YiXue ZaZhi. 2003;83:1122-1125. [PubMed] |
| 4. | Liu YK, Shen W. Inhibitive effect of cordyceps sinensis on experimental hepatic fibrosis and its possible mechanism. World J Gastroenterol. 2003;9:529-533. [PubMed] |
| 5. | Nishio A, Keeffe EB, Gershwin ME. Immunopathogenesis of primary biliary cirrhosis. Semin Liver Dis. 2002;22:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7-G11. [PubMed] |
| 7. | Lee M, Song SU, Ryu JK, Suh JK. Sp1-dependent regulation of the tissue inhibitor of metalloproteinases-1 promoter. J Cell Biochem. 2004;91:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Yu YY, Wang QH, Zhu LM, Zhang QB, Xu DZ, Guo YB, Wang CQ, Guo SH, Zhou XQ, Zhang LX. A clinical research on oxymatrine for the treatment of chronic hepatitis B. Zhonghua GanZangBing ZaZhi. 2002;10:280-281. [PubMed] |
| 9. | Liu J, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials. Am J Gastroenterol. 2003;98:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Li J, Zeng M, Lu L, Qu D, Mao Y, Fan Z, Hua J. The inhibitory effect of oxymatrine on hepatitis C virus in vitro. Zhonghua GanZangBing ZaZhi. 2001;9 Suppl:12-14. [PubMed] |
| 11. | Song J, Wang LL, Zhu L, Zhong HM, Yao P. Effects of oxymatrine on procollagen metabolism and its gene expression in experimental fibrotic rats. Zhonghua GanZan Bing ZaZhi. 2003;11:697. [PubMed] |
| 12. | Hellemans K, Rombouts K, Quartier E, Dittié AS, Knorr A, Michalik L, Rogiers V, Schuit F, Wahli W, Geerts A. PPARbeta regulates vitamin A metabolism-related gene expression in hepatic stellate cells undergoing activation. J Lipid Res. 2003;44:280-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Murphy F, Waung J, Collins J, Arthur MJ, Nagase H, Mann D, Benyon RC, Iredale JP. N-Cadherin cleavage during activated hepatic stellate cell apoptosis is inhibited by tissue inhibitor of metalloproteinase-1. Comp Hepatol. 2004;3 Suppl 1:S8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67-E70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Xiang X, Wang G, Cai X, Li Y. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J (Engl). 2002;115:593-596. [PubMed] |