Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.249
Revised: June 28, 2004
Accepted: July 17, 2004
Published online: January 14, 2005
AIM: High levels of serum sialyl Lewisa (sLea) are frequently found in cholangiocarcinoma (CCA) patients and have been suggested to be a serum marker for CCA. However, the significance of this antigen in CCA is unknown. In this study, the clinical significance of sLea expression in CCA tissues and the possible role of sLea in vascular invasion in vitro were elucidated.
METHODS: Expression of sLea in tumor tissues of 77 patients with mass-forming CCA and 33 with periductal infiltrating CCA was determined using immunohistochemistry. The in vitro assays on adhesion and transmigration of CCA cells to human umbilical vein endothelial cells were compared between CCA cell lines with and without sLea expression.
RESULTS: sLea was aberrantly expressed in 60% of CCA tumor tissues. A significant relationship was found between the frequency of sLea expression and the mass-forming type CCA (P = 0.041), well differentiated histological grading (P = 0.029), and vascular invasion (P = 0.030). Patients with positive sLea expression had a significantly poorer prognosis (21.28 wk, 95% CI = 16.75-25.81 wk) than those negative for sLea (37.30 wk, 95% CI = 27.03-47.57 wk) (P<0.001). Multivariate analysis with adjustment for all covariates showed that patients positive for sLea possessed a 2.3-fold higher risk of death than patients negative for sLea (P<0.001). The role of sLea in vascular invasion was demonstrated using in vitro adhesion and transmigration assays. KKU-M213, a human CCA cell-line with a high expression of sLea, adhered and transmigrated to IL-1β-activated endothelial cells of the human umbilical vein more than KKU-100, the line without sLea expression (P<0.001). These processes were significantly diminished when the antibodies specific to either sLea or E-selectin were added to the assays (P<0.001).
CONCLUSION: This study demonstrates the clinical significance of sLea expression in vascular invasion, and an unfavorable outcome in CCA. The role of sLea in vascular invasion which may lead to poor prognosis is supported by the in vitro adhesion and transmigration studies.
- Citation: Juntavee A, Sripa B, Pugkhem A, Khuntikeo N, Wongkham S. Expression of sialyl Lewisa relates to poor prognosis in cholangiocarcinoma. World J Gastroenterol 2005; 11(2): 249-254
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.249
Metastasis spreads malignant cells from a primary tumor throughout the body resulting in growth of secondary tumors in other tissues or organs. The ability of disseminated cancer cells to re-establish themselves is regulated by a combination of factors, including access to microvasculature and host-tumor cell interaction[1]. Attachment to vascular endothelia is the start of the metastatic cascade and evidence suggests that attachment precedes, and is required for, tumor cell extravasation and subsequent invasion into the target organ parenchyma[2]. Organ-specific receptors have been identified on the luminal surface of microvascular endothelia, specifically recognized by tumor cell ligands, thereby facilitating tumor cell arrest and transmigration into the extravascular space.
Sialyl Lewisa (sLea) antigen, discovered by Koprowski et al[3] with the use of monoclonal antibody CA19-9, is a tetrasaccharide epitope (sialylated lacto-N-fucopentaose II) on the tumor cell membrane which may have a role in cancer dissemination[4,5]. There is evidence that sLea expressed on tumor cells plays an important role in the adhesion of tumor cells to E-selectin on endothelial cells in the extravasation process[6,7]. Detection of sLea, in either tissue or pre- and post-operative serum is a prediction of increased cancer mortality[8]. The association of high levels of serum sLea with tumor invasion is common in cancer patients[5,9-11].
Cholangiocarcinoma (CCA), a bile duct cancer, is highly prevalent in Northeast Thailand[12]. Early stage CCA often goes undetected, most patients are diagnosed at an advanced or disseminated stage with a poor prognosis. High levels of serum sLea are frequently found in CCA patients and have been suggested to be a serum marker for CCA[13-17]. However, the role of sLea in CCA is unclear. We therefore evaluated the association of sLea expression in tumor tissues with the clinico-pathology and survival of CCA patients. The role of sLea antigen in the adhesion and transmigration of human CCA cells to human umbilical vein endothelial cells (HUVEC) in vitro was demonstrated.
Surgical specimens of 110 CCAs were obtained from the files of the Liver Fluke and Cholangiocarcinoma Research Center, Khon Kaen University, Thailand. The specimens were classified into 2 types: 77 cases of mass forming CCA and 33 cases of periductal infiltrating CCA. The mean age of patients was 55 years (range, 32 to 75 years). Seventy-two were males, and 31 were females. Informed consent was obtained from each subject and the Human Research Ethics Committee, Khon Kaen University, approved our research protocol. Cancer diagnosis was verified by histology with UICC TNM classification. Clinical follow-up was available for 104 (94.5%) of the patients. Survival of each CCA patient was recorded after surgery until May 15, 2001. Ninety-one patients (82.7%) died by the end of the follow-up period.
The two human cholangiocarcinoma cell-lines used (KKU-M213 and KKU-100) were from the Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Thailand. The human umbilical vein endothelial cells (HUVECs) were from the American Type Culture Collection (Manassas, VA).
CCA cells were cultured in a HAM-F12 medium (Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL fungizone. sLea expression in CCA cell-lines was determined by immunocytochemistry using anti-CA19-9 antibody (Novocastra, Newcastle upon Tyne, UK). sLea was highly expressed in KKU-M213, more than 95% of cells had strong, positive staining. In contrast, KKU-100 cells showed no reaction (data not shown).
All specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 4-μm-thick serial sections for immunohistochemical staining using avidin-biotin complex technique. Briefly, the paraffin sections were deparaffinized, hydrated and endogenous peroxidase-blocked with hydrogenperoxide. After non-specific staining was blocked with normal horse serum, the sections were incubated with 1:100 anti-sLea (anti CA19-9) overnight, followed by biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, CA) and streptavidin-peroxidase (Vector). After washed, the sections were developed in 0.05% 3,3’-diaminobenzidine tetrahydrochloride (DAB; Sigma Chemical Co., St Louis, MO), counterstained with hematoxylin, dehydrated, cleared and mounted. When PBS was applied instead of the primary antibody, there was no positive staining.
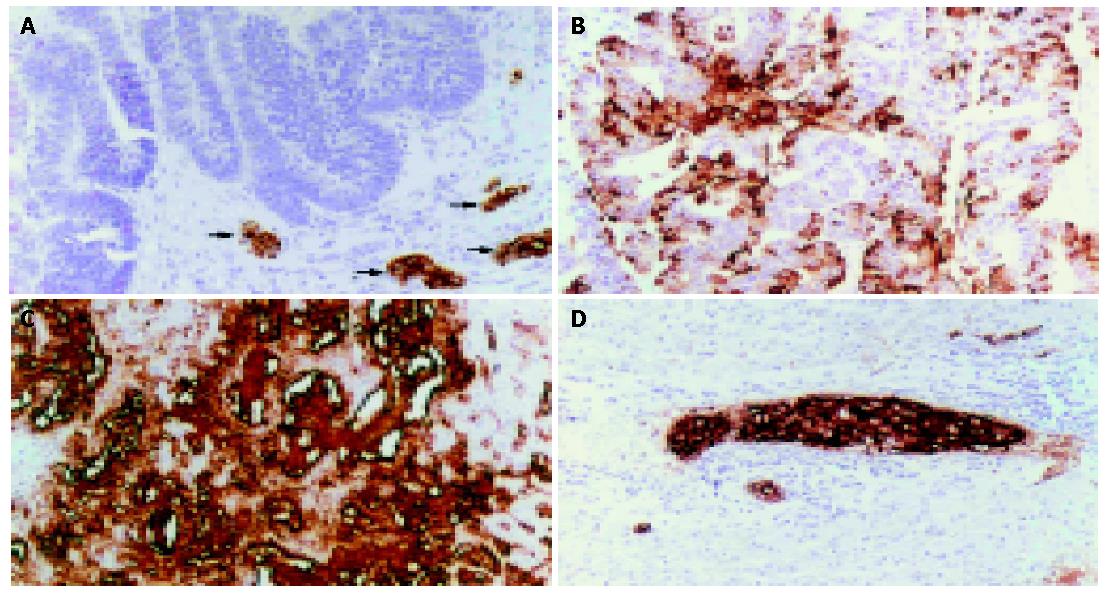
The intensity of sLea expression was semi-quantitatively classified into 4 groups on the basis of the percentage of positive tumor cells: 0%, negative; 1-25%, +1; 26-50%, +2 and >50%, +3.
The adhesion assay procedure[18] was as follows. In briefly: HUVECs (2 to 5 passages) were grown in Kaighn’s F12K medium, supplemented with endothelial cell growth supplement (Life Technologies, Rockville, MD) and seeded at 4×104 cells/well in a 96-well plate, pre-coated with 0.1% gelatin. The plate was then incubated at 37 °C in an atmosphere containing 50 mL/L CO2 for 24 h. After activation of the rIL-1β (100 U/mL) (Life Technologies, Rockville, MD) for 4 h, the medium was removed and the cells were blocked with 1% bovine serum albumin complete media for 1 h.
Cell suspensions of KKU-M213 or KKU-100 (2×104 cells), in phosphate-buffered saline (PBS) with 1 mmol/L CaCl2, were added to the HUVEC in each well and incubated for further 45 min. Unbound cells were removed by washing the wells with PBS. Adhered cells were fixed for 15 min with 2.5% glutaraldehyde, then stained with an antibody of 1:400 of pan-cytokeratin (Novocastra Lab, Newcastle upon Tyne, UK) and 1:100 horseradish peroxidase-conjugated goat anti-mouse IgG (Zymed Laboratories, South San Francisco, CA).
The cells that adhered to the HUVECs were counted by microscopy in nine low power fields (×100 magnification). Non-stimulated HUVECs were used as the controls. Triplicate assays were performed and at least two separate experiments were done.
A modified transmigration in vitro assay was performed as per Yoshida[19]. Approximately 8×104 HUVECs were plated on a 0.3-mg pre-coated-Matrigel-culture insert (Becton-Dickinson, San Jose, CA). The monolayer was activated with 100 U/mL IL-1β for 4 h. After blocked with 1% bovine serum albumin complete media for 1 h, 4×104 cells of KKU-M213 cells in PBS with 1 mmol/L CaCl2 were added to each insert and incubated for 30 min. Cells on the upper face of the membrane were scraped using a cotton swab and cells on the lower face were fixed with 25% methanol for 15 min and stained with 0.5% crystal violet in 25% methanol. The number of migrated cells on the lower face of the filter was counted under microscopy in nine fields (×100 magnification). KKU-M213 cells incubated in the pre-coated-Matrigel insert without HUVECs were used as control. Assays were done in triplicate and repeated at least twice.
KKU-M213 cells were incubated with 50 μg/mL of anti-sLea monoclonal antibody (Chemicon International, Temecula, CA) for 30 min prior to the adhesion or transmigration assays. For HUVEC, a monolayer of activated HUVEC was pre-incubated with 10 μg/mL anti-human E-selectin (Santa Cruz Biotechnology, Santa Cruz, CA) at 37 °C for 15 min[7]. The excess anti-E-selectin was washed out with PBS before incubated with tumor cells in the adhesion or transmigration assays. The viability of treated cells determined using trypan blue exclusion dye was 96.86%, which was not significantly different from that of the non-treated sample.
Data were presented as mean±SD. The Student t test was used for comparisons and P<0.05 was considered statistically significant. The association of two categorical variables was analyzed by the χ2-test or Fisher’s exact probability test.
Survival of the patients was compared between the group with positive sLea antigen expression and the group with negative sLea antigen expression according the Kaplan-Meier method. The significance of the difference in survival between the 2 groups was tested by the log-rank test. Several clinico-pathologic factors were subjected to univariate and multivariate analysis using the Cox proportional-hazard regression model. The relative risk of death was compared using the assessment of hazard ratio. Differences were considered statistically significant at P<0.05.
By immunohistochemistry, sLea was constitutively expressed in normal biliary epithelial cells. It was localized at the apical surface, cytoplasm and/or stroma of CCA tissues (Figure 1). The expression of sLea was detected in 60% (66/110) of CCA patients. There were 79% (26/33) of periductal infiltrating CCA patients and 52% (40/77) of mass-forming CCA patients who expressed sLea (P = 0.015) (Table 1).
| Expression of sLea | Tumor type | Total | |
| Mass-forming | Periductal infiltraing | ||
| 0 | 37 | 7 | 44 |
| 1+ | 7 | 7 | 14 |
| 2+ | 8 | 6 | 14 |
| 3+ | 25 | 13 | 38 |
| Total | 77 | 33 | 110 |
The association of sLea expression in CCA patients with clinico-pathologic features was determined. Three variables, mass-forming CCA, well differentiated adenocarcinoma histological grading and vascular invasion were statistically significant and were associated with the expression of tumor sLea (Table 2).
| Expression of sLea | P | |||||
| 0 | 1+ | 2+ | 3+ | |||
| CCA type | ||||||
| Mass-forming | 37 | 7 | 8 | 25 | 0.041 | |
| Periductal-infiltrating | 7 | 7 | 6 | 13 | ||
| Histology type | ||||||
| Papillary | 2 | 3 | 1 | 8 | 0.029 | |
| Well differentiated | 8 | 5 | 6 | 14 | ||
| Moderately differentiated | 8 | 3 | 4 | 5 | ||
| Poorly differentiated | 19 | 2 | 0 | 9 | ||
| Squamous/adenosquamous | 7 | 1 | 3 | 2 | ||
| Vascular invasion | - No | 13 | 8 | 9 | 11 | 0.03 |
| - Yes | 31 | 6 | 5 | 21 | ||
| Neural invasion | - No | 27 | 8 | 4 | 14 | 0.054 |
| - Yes | 17 | 6 | 10 | 24 | ||
| Lymphatic invasion | - No | 9 | 5 | 2 | 9 | 0.56 |
| - Yes | 35 | 9 | 12 | 29 | ||
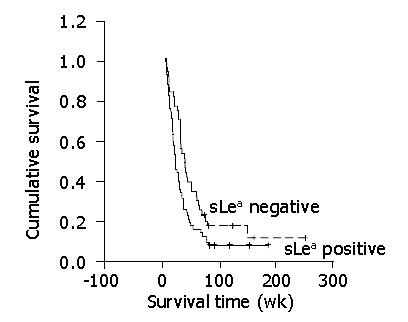
Median overall survival in CCA patients with positive and negative sLea expressions was 21.28 wk (95% CI = 16.75-25.81) and 37.30 wk (95% CI = 27.03-47.57), respectively. The survival rate of the patients with positive sLea expression was significantly poorer than that of the patients with negative sLea expression (P = 0.021, log-rank test, Figure 2). Well differentiated type CCA (P = 0.027) and the expression of sLea (P = 0.001) were independently poor prognostic indicators contributing to disease-free survival of CCA (Table 3).
| Variable | Coefficient | SE | Hazard ratio | P |
| Age (55 vs >55 yr) | -0.013 | 0.011 | 0.987 | 0.257 |
| Sex (male vs female) | 0.294 | 0.25 | 1.342 | 0.239 |
| CCA type (mass-forming vs periductal-infiltrating) | 0.44 | 0.322 | 1.552 | 0.173 |
| Histology | ||||
| Papillary | -0.83 | 0.471 | 0.436 | 0.078 |
| Well differentiated | -0.891 | 0.402 | 0.41 | 0.027 |
| Moderately differentiated | -0.736 | 0.462 | 0.479 | 0.111 |
| Poorly differentiated | -0.356 | 0.405 | 0.701 | 0.379 |
| Squamous/adenosquamous | 0.264 | 0.756 | 1.302 | 0.727 |
| Vascular invasion (present vs absent) | -0.234 | 0.272 | 0.791 | 0.39 |
| Neural invasion (present vs absent) | -0.362 | 0.247 | 0.696 | 0.143 |
| Lymphatic invasion (present vs absent) | 0.053 | 0.313 | 1.055 | 0.865 |
| Tissue sLea (present vs absent) | 0.834 | 0.26 | 2.302 | 0.001 |
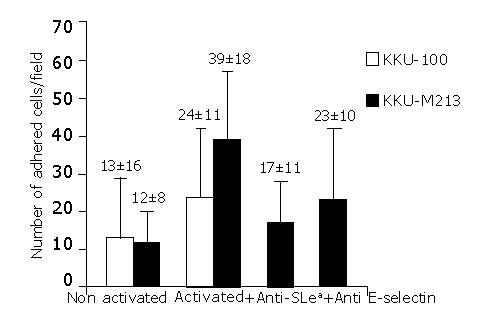
The adhesion of CCA cells with high positive sLea expression (KKU-M213) was compared to that of CCA cells with negative sLea expression (KKU-100). The basal adhesion level of these two cell-lines to non-activated endothelial cells was not significantly different. However, in the cytokine-activated HUVECs, the adhesion of KKU-M213 cells was significantly greater than that of KKU-100 cells (P<0.001) (Figure 3). This finding was confirmed by the inhibition assay in which tumor cells were pre-treated with monoclonal antibodies for sLea before added to the HUVEC. The number of cancer cells that adhered to the rIL-1β activated HUVECs decreased to base levels, and was significantly less than that without anti-sLea (P<0.001) (Figure 3).
To evaluate the contribution of E-selectin to the adhesion of CCA cells, adhesion assays of KKU-M213 to HUVECs were performed with and without rIL-1β-activation. rIL-1β had a clear stimulatory effect on the adhesion of cancer cells to HUVECs (Figure 3). The adhesion of KKU213 to rIL-1β-activated HUVECs was about 4-fold greater than that without activation (P<0.001). The significant contribution of E-selectin was confirmed by showing that incubation of the activated HUVECs with monoclonal antibody to E-selectin, before addition of cancer cells, clearly inhibited cell adhesion (Figure 3) (P<0.001). However, the number of adhered cells was still greater than the basal level (P<0.001).
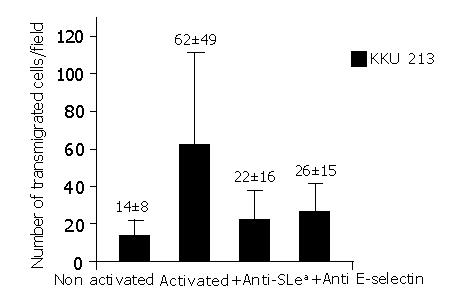
The contributions of sLea and E-selectin to transmigration of KKU-M213 via the HUVECs were evident. The number of cancer cells that transmigrated through HUVEC was significantly reduced, when prior to the transmigration assay. The KKU-M213 cells were treated with anti-sLea (P<0.001) or the activated HUVECs were pre-treated with anti-E-selectin (P<0.001) (Figure 4). This observation was contributed mainly via transmigration of KKU-213 through the HUVECs since no KKU-213 cells or HUVECs migrated through the control insert within 30 min of incubation.
The present study demonstrated that 60% of CCA tumor tissues were aberrantly expressed sLea. The univariate analysis revealed mass-forming type CCA, well-differentiated histological type and presence of vascular invasion tumor associated with the expression of tumor sLea. Tsuji et al[15] and Minato et al[20] supported our immunohistology results that sLea was expressed in 60% of intrahepatic CCAs and the expression of sLea antigen was more frequent in well-differentiated adenocarcinoma cells. In addition, the high level of serum sLea (CA19-9) in CCA patients was recently demonstrated to be related to venous invasion, perineural invasion and lymph node metastases[14].
The multivariate analysis indicated the expression of tumor sLea as an independent prognostic factor affecting disease-free survival and overall survival. From our literature search, this appears to be the first report on the association of sLea expression with poor prognosis in CCA. Patients with positive sLea in tumor tissues had significantly shorter survival than those with negative sLea. Therefore, the presence of tumor sLea can be used as a prognostic risk factor related to survival of CCA patients and may help select patients with poor prognoses that can then be offered adjuvant therapy.
A key event in cancer metastasis is the transendothelial migration of tumor cells. This process involves multiple adhesive interactions between tumor cells and the endothelium. After adhering to the surface of endothelial cells, tumor cells must penetrate the endothelial junction. The contribution of sLea to the adhesion of tumor cells to endothelial cells via E-selectin has been observed in various cancer cell-lines[21-23]. In the present study, the contribution of sLea to vascular invasion was demonstrated not only by a statistical association analysis but also in the in vitro adhesion and transmigration assays of CCA cells to E-selectin-mediated human endothelial cells.
The role of sLea in endothelial cell adhesion was assessed by comparing the adhesion levels of two CCA cell lines: one with a high expression of sLea (KKUM213) and one with undetectable sLea (KKU-100). These two cell lines had comparable basal adhesion to non-activated HUVECs. However, upon rIL-1β-activation, the number of cells adhering to the activated-HUVECs of KKU-M213 was significantly greater than that of KKU-100.
Our in vitro studies with KKU-M213 and KKU-100 had a low, but measurable basal adhesion to non-activated endothelial cells. Blocking-activated HUVECs with antibody to E-selectin did not completely keep adhesion at a basal level, suggesting the involvement of other, as yet unknown, carbohydrate ligands on CCA cells, and/or receptors on activated HUVECs, in the adhesion of these cell-lines.
The importance of sLea to allow or enable attachment of CCA cells to endothelial cells has therefore been confirmed by the selective blocking of sLea (marked reduction of adhesion of KKU-M213) by specific antibodies. Moreover, the treatment inhibited the binding of KKU-M213 to activated HUVECs close to the basal level obtained from KKU-M213, with non-activated HUVECs. The observation indicates that the KKU-M213 cells adhering to the activated HUVECs was mainly via sLea. The same conclusion is drawn from the transmigration study.
Blocking either sLea or E-selectin, with a specific neutralizing antibody, can inhibit adhesion and transmigration of CCA cells to endothelial cells, confirming the involvement of sLea and E-selectin in these processes.
The sLea antigen is expressed at trace levels in normal biliary cells but is expressed in a high level in tumor cells and can be detected as a tumor marker in serum. The increased serum level of sLea in CCA has been reported ranging from 57 to 100%[13-16]. The discrepancies between various investigators might be due to the differences in etiology and incidence[24-29].
Several lines of evidence and our results point to the cancer-associated carbohydrate antigen, sLea, in the vascular invasion via the adhesion and transmigration of cancer cells to vascular endothelial cells. It may also contribute to the hematogenous metastasis of cancer and unfavorable outcome. sLea is immunogenic and potentially a target for passive-and active- specific immunotherapy for human cancers in which the sLea antigen could be expressed as a tumor-differentiation antigen[30]. CCA is a highly metastatic cancer with a poor prognosis. The association of sLea expression with a poor prognosis in CCA and the contribution of sLea to CCA-cell adhesion and transmigration via E-selectin-mediated HUVECs demonstrated in this study suggest the possible use of this ligand as a target for specific immunotherapy of CCA in the prevention of metastases, especially in patients with aberrant expression of sLea.
The authors thank Professor James Will, University of Wisconsin-Madison, for assistance with the English-language presentation of the manuscript.
Co-first-authors: Apa Juntavee and Sopit Wongkham
Co-correspondents: Banchob Sripa
Edited by Wang XL
| 1. | Radinsky R, Fidler IJ. Regulation of tumor cell growth at organ-specific metastases. In Vivo. 1992;6:325-331. [PubMed] |
| 2. | Brodt P. Adhesion recrptors and proteolytic mechanisms in cancer invasion and metastasis. Berlin: Springer-Verlag 1996; 167-242. |
| 3. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 865] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Zenita K, Kirihata Y, Kitahara A, Shigeta K, Higuchi K, Hirashima K, Murachi T, Miyake M, Takeda T, Kannagi R. Fucosylated type-2 chain polylactosamine antigens in human lung cancer. Int J Cancer. 1988;41:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Kannagi R, Kitahara A, Itai S, Zenita K, Shigeta K, Tachikawa T, Noda A, Hirano H, Abe M, Shin S. Quantitative and qualitative characterization of human cancer-associated serum glycoprotein antigens expressing epitopes consisting of sialyl or sialyl-fucosyl type 1 chain. Cancer Res. 1988;48:3856-3863. [PubMed] |
| 6. | Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 977] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354-361. [PubMed] |
| 8. | Weston BW, Hiller KM, Mayben JP, Manousos G, Nelson CM, Klein MB, Goodman JL. A cloned CD15s-negative variant of HL60 cells is deficient in expression of FUT7 and does not adhere to cytokine-stimulated endothelial cells. Eur J Haematol. 1999;63:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, Rake B, Space S, Westrick B, Schoemaker H. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29:549-552. [PubMed] |
| 10. | Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983;43:5489-5492. [PubMed] |
| 11. | Lowe JB, Stoolman LM, Nair RP, Larsen RD, Berhend TL, Marks RM. ELAM-1--dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell. 1990;63:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 523] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Vatanasapt V, Tangvoraphonkchai V, Titapant V, Pipitgool V, Viriyapap D, Sriamporn S. A high incidence of liver cancer in Khon Kaen Province, Thailand. Southeast Asian J Trop Med Public Health. 1990;21:489-494. [PubMed] |
| 13. | Torzilli G, Makuuchi M, Ferrero A, Takayama T, Hui AM, Abe H, Inoue K, Nakahara K. Accuracy of the preoperative determination of tumor markers in the differentiation of liver mass lesions in surgical patients. Hepatogastroenterology. 2002;49:740-745. [PubMed] |
| 14. | Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, Abu-Elmaagd K, Madariaga JR, Slivka A. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002;56:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Tsuji M, Kashihara T, Terada N, Mori H. An immunohistochemical study of hepatic atypical adenomatous hyperplasia, hepatocellular carcinoma, and cholangiocarcinoma with alpha-fetoprotein, carcinoembryonic antigen, CA19-9, epithelial membrane antigen, and cytokeratins 18 and 19. Pathol Int. 1999;49:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427-432. [PubMed] |
| 17. | Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 285] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Aubert M, Panicot L, Crotte C, Gibier P, Lombardo D, Sadoulet MO, Mas E. Restoration of alpha(1,2) fucosyltransferase activity decreases adhesive and metastatic properties of human pancreatic cancer cells. Cancer Res. 2000;60:1449-1456. [PubMed] |
| 19. | Yoshida M, Chien LJ, Yasukochi Y, Numano F. Differentiation-induced transmigration of HL60 cells across activated HUVEC monolayer involves E-selectin-dependent mechanism. Ann N Y Acad Sci. 2000;902:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Minato H, Nakanuma Y, Terada T. Expression of blood group-related antigens in cholangiocarcinoma in relation to non-neoplastic bile ducts. Histopathology. 1996;28:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Takada A, Ohmori K, Takahashi N, Tsuyuoka K, Yago A, Zenita K, Hasegawa A, Kannagi R. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochem Biophys Res Commun. 1991;179:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Tyrrell D, James P, Rao N, Foxall C, Abbas S, Dasgupta F, Nashed M, Hasegawa A, Kiso M, Asa D. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci USA. 1991;88:10372-10376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Yoon WH, Park HD, Lim K, Hwang BD. Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem Biophys Res Commun. 1996;222:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Lindberg B, Arnelo U, Bergquist A, Thörne A, Hjerpe A, Granqvist S, Hansson LO, Tribukait B, Persson B, Broomé U. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy. 2002;34:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology. 2003;50:1669-1674. [PubMed] |
| 26. | Torok N, Gores GJ. Cholangiocarcinoma. Semin Gastrointest Dis. 2001;12:125-132. [PubMed] |
| 27. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Ahrendt SA, Pitt HA, Nakeeb A, Klein AS, Lillemoe KD, Kalloo AN, Cameron JL. Diagnosis and management of cholangiocarcinoma in primary sclerosing cholangitis. J Gastrointest Surg. 1999;3:357-367; discussion 367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Hultcrantz R, Olsson R, Danielsson A, Järnerot G, Lööf L, Ryden BO, Wahren B, Broomé U. A 3-year prospective study on serum tumor markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 1999;30:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Ravindranath MH, Amiri AA, Bauer PM, Kelley MC, Essner R, Morton DL. Endothelial-selectin ligands sialyl Lewis(x) and sialyl Lewis(a) are differentiation antigens immunogenic in human melanoma. Cancer. 1997;79:1686-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |