Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.237
Revised: May 12, 2004
Accepted: June 18, 2004
Published online: January 14, 2005
AIM: Irritable bowel syndrome (IBS) is a functional bowel disorder. Its major symptom is bowel dysmotility, yet the mechanism of the symptom is poorly understood. Since the neurokinin-1 receptor (NK1R)-mediated signaling in the gut is important in the control of normal bowel motor function, we aimed to investigate whether the NK1R-mediated bowel motor function was altered in IBS, using a rat IBS model that was previously reported to show colonic dysmotility in response to restraint stress.
METHODS: IBS symptoms were produced in male Sprague-Dawley rats by inducing colitis with acetic acid. Rats were left to recover from colitis for 6 d, and used for experiments 7 d post-induction of colitis. Motor activities of distal colon were recorded in vitro.
RESULTS: The contractile sensitivity of isolated colon to a NK1R agonist [Sar9,Met(O2)11]-substance P (1-30 nmol/L) was higher in IBS rats than that in normal rats. After the enteric neurotransmission was blocked by tetrodotoxin (TTX, 1 μmol/L), the contractile sensitivity to the NK1R agonist was increased in normal colon but not in IBS rat colon. The NK1R agonist-induced contraction was not different between the two groups when the agonist was challenged to the TTX-treated colon or the isolated colonic myocytes. A nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μmol/L) augmented the NK1R agonist-induced contraction only in normal rat colon.
CONCLUSION: These results suggest that the NK1R-meidated colonic motor response is increased in IBS rats, due to the decrease in the nitrergic inhibitory neural component.
- Citation: La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol 2005; 11(2): 237-241
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.237
IBS is a functional bowel disorder, and its major clinical symptom is disordered defecation associated with abdominal pain/discomfort[1,2]. The disordered defecation can be diarrhea or constipation, or an alternating bowel habit from one to the other over time[3]. Based on the disordered defecation patterns, patients diagnosed with IBS have been divided into different subtypes such as diarrhea-predominant IBS or constipation-predominant IBS[4]. It has been suggested that the disordered defecation in IBS patients results from abnormal motor function of the colon[5-8]. However, the mechanisms underlying the disordered defecation in IBS are still poorly understood.
Researchers have consistently reported that substance P (SP) is an important enteric transmitter in the control of bowel motility[9]. Interacting mainly with the neurokinin-1 receptor (NK1R), SP can both stimulate and inhibit bowel motility by direct activation of the muscle cells and stimulation of enteric neural circuits[10]. Thus, it is highly conceivable that alterations in the NK1R-mediated signaling can cause bowel dysmotility. Indeed, pathophysiological involvement of NK1R has been shown in inflammation or stress-induced colonic dysmotility[11-14].
Considering the importance of the NK1R-mediated signaling in normal bowel motility, one can hypothesize that the disordered defecation in IBS might be related to a disturbance in the NK1R-mediated control of colonic motility. We aimed to test this hypothesis using an animal model of IBS. Previously we reported that rats developed IBS symptoms after subsidence of acetic acid-induced colitis[15]. This animal model showed a visceral hypersensitivity and an altered defecation pattern in the absence of histological and biochemical signs of intestinal inflammation. In the colon of this rat model of IBS, we investigated whether the NK1R-mediated motor response was altered.
Male Sprague-Dawley rats, weighing 270-310 g, were housed in stainless steel hanging cages in a colony room maintained under a 12 h light/dark cycle with a room temperature of 22±1 °C and a humidity of 65-70%. Water and food were available ad libitum. IBS symptoms were produced as described previously[15]. Briefly, colitis was induced by intracolonic instillation of 1 mL 4% acetic acid. Control animals received saline instead of acetic acid. Rats were left to recover from colitis for 6 d, and used for experiments 7 d post-induction of colitis.
Motor activity of isolated colonic segment On the day of experiments, rats were killed by cervical dislocation, and a 2 cm distal colonic segment was removed. The segment was suspended in a 20 mL organ bath containing oxygenated (95% O2 and 50 mL/L CO2) Krebs solution maintained at 37 °C. The distal end of the segment was tied around the mouth of J-tube that was connected via a 3-way connector to a syringe and to a pressure transducer (RP-1500, Narco Bio-systems Inc., USA). The proximal end of the segment was ligated with a thread that was connected to an isometric force displacement transducer (FT-03, Grass-Telefactor, USA). The signals from both transducers were acquired by PowerLab/400 (AD Instruments, Castle Hill, Australia) and recorded on an IBM-compatible computer.
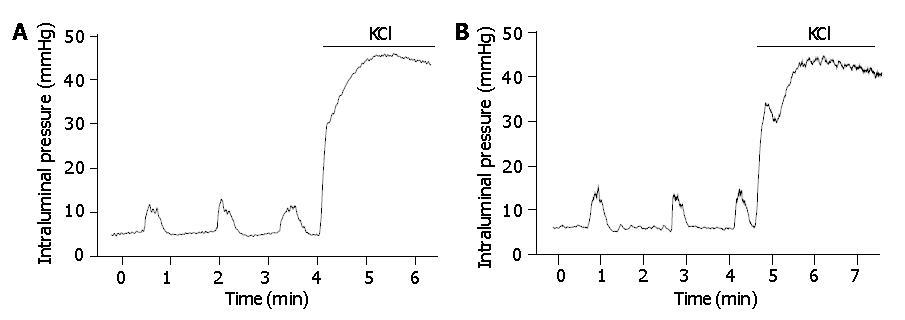
Initial 1-g tension was loaded on the colonic segment and the lumen of the segment was filled with a 0.2 mL Krebs solution per 1 cm length of the segment. The mechanical activities of the colonic segment were detected as changes in intraluminal pressure. This parameter was reported to reflect the motor activity of intestinal circular muscles[16]. After a 60-min equilibration period, drugs were cumulatively administered into the bath with a 5-min exposure time at each concentration. The effect of a drug on the colonic motor activity was quantified by measuring the mean intraluminal pressure at a given concentration. The mean intraluminal pressure was digitally calculated by dividing an integral value of pressure (area under the pressure trace) by the number of data points (tracing time). At the end of each experiment, the tonic contraction by KCl (60 mmol/L) was measured to normalize the motor activity of the isolated colon at each concentration of drugs (% of the maximal amplitude of the KCl-induced tonic contraction, % KCl).
Smooth muscle layers from the colon were isolated, cut into small pieces and placed in nominal Ca2+-free physiological salt solution (Ca2+-free PSS). These segments were incubated in a medium modified from Kraft-Brühe (KB) medium[17] for 30-60 min at room temperature. They were then incubated for 20-30 min at 37 °C in digestion medium (Ca2+-free PSS) containing 1.5 mg/mL collagenase type 2, 2.0 mg/mL trypsin inhibitor, 2.0 mg/mL bovine serum albumin and 0.5 mg/mL dithioerythritol. After digestion, the supernatant was discarded, the softened muscle segments were transferred again into modified KB medium, and single cells were dispersed by gentle agitation with a wide-bore glass pipette. Isolated colonic myocytes were kept in modified KB medium at 4 °C until use. All experiments were carried out at room temperature within 12 h of harvesting cells.
Isolated colonic myocytes were transferred to a stage on an inverted microscope (Olympus CK2, Japan) and allowed to stick lightly to the glass coverslip bottom of a small chamber for 10 min. The cells were then perfused with physiological salt solution (PSS) to remove cellular debris. Single smooth muscle cells were identified and cell image was digitally captured using CCD camera (TMC-7, PULNiX Inc., USA) at 0.05 frame per second (fps) rate. Cell length was measured using software for image analysis. Cell contraction was expressed as a percent decrease in cell length by a drug from control length (the length of cells before the application of drugs).
The Krebs solution contained (in mmol/L) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11 glucose. The Ca2+-free PSS contained (in mmol/L) 135 NaCl, 5 KCl, 1.2 MgCl2, 10 glucose, and 10 HEPES (adjusted to pH 7.4 with Tris). PSS contained (in mmol/L) 135 NaCl, 5 KCl, 2 CaCl2, 10 glucose, and 10 HEPES. [Sar9, Met(O2)11]-substance P (SP) was purchased from Tocris Cookson (Avonmouth, UK). Collagenase type 2 was purchased from Worthington (Lakewood, NJ, USA). All the following chemicals were purchased from Sigma (St. Louis, MO, USA): tetrodotoxin (TTX), Nω-nitro-L-arginine methyl ester (L-NAME), dithioerythritol, trypsin inhibitor.
Data were expressed as mean±SE with n, the number of animals. Unpaired Student’s t-test was used for statistical comparison (at P<0.05 significance level). In case of analyzing the effect of a NK1R agonist on normal and IBS colon before and after TTX-treatment, the significance level was adjusted using Bonferroni procedure.
The isolated colonic segments showed spontaneous motor activities in rest, and tonically contracted under a high KCl (60 mmol/L) solution (Figure 1). There was no difference in the KCl-induced contraction (normalized by dividing by wet weight of the colonic segment) between normal and IBS rat colons (329±31 mmHg/g vs 326±37 mmHg/g, P>0.95, n = 10). The frequency of the spontaneous phasic contraction was 0.79±0.08 beat per minute (BPM) (n = 10) in normal and 0.77±0.07 BPM (n = 10) in IBS rat colon. The amplitude of the spontaneous contraction was 13.1±1.6% KCl and 13.3±1.2% KCl in normal rat colon and IBS rat colon, respectively (P>0.9).
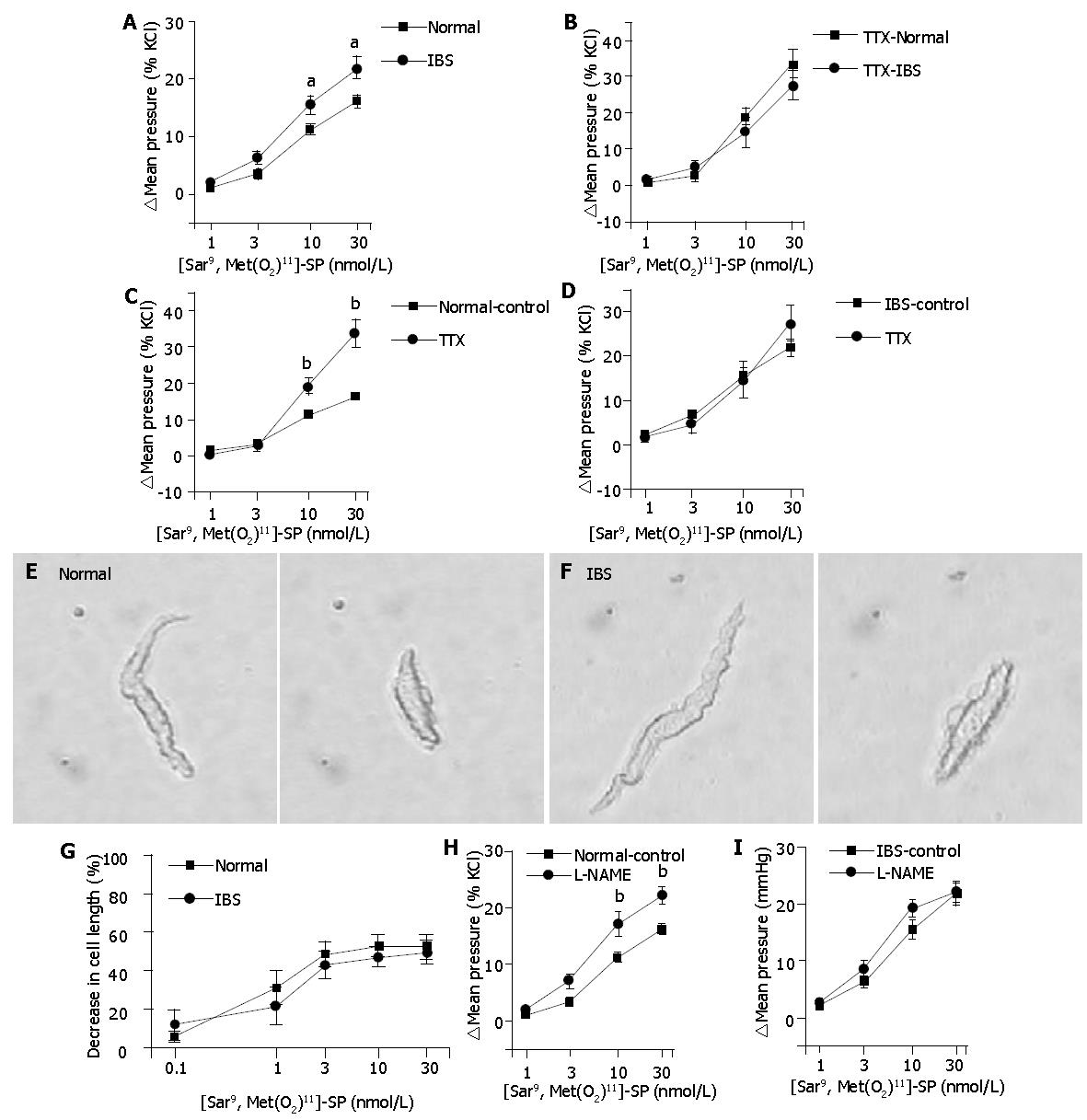
As shown in Figure 2A, a selective NK1R agonist, [Sar9,Met(O2)11]-substance P (SP), contracted the circular muscle of an isolated distal colonic segment. The contractile effect of [Sar9,Met(O2)11]-SP was more prominent in IBS rat colon than that in normal rat colon at 10 and 30 nmol/L (P<0.05).
Blocking the enteric neurotransmission with TTX (1 μmol/L) increased the resting mean intraluminal pressure by 9.7±1.1% KCl (n = 8) in normal rat colon and by 8.0±1.7% KCl (n = 7) in IBS rat colon (P = 0.4). The contractile effect of [Sar9,Met(O2)11]-SP was not different between the two groups under the presence of TTX (Figure 2B). In the TTX-pretreated normal rat colon (n = 8), the contractile effect of [Sar9,Met(O2)11]-SP was increased to 19.0±2.2% KCl (P<0.01 vs 11.2±0.9% KCl) and to 33.5±3.8% KCl (P<0.01 vs 16.3±0.9% KCl) at 10 and 30 nmol/L, respectively (Figure 2C), which implied that an inhibitory neural component was involved in the NK1R agonist-induced contraction in normal rat colon. In IBS rat colon (n = 7), the contraction at each concentration of [Sar9,Met(O2)11]-SP was not significantly changed by TTX pretreatment (Figure 2D).
The initial length of isolated colonic myocytes was 77.8±3.2 μm (n = 7) and 70.5±2.7 μm (n = 8) in normal and IBS groups, respectively (P>0.09). [Sar9,Met(O2)11]-SP concentration-dependently decreased the length of isolated muscle cells. At the highest concentration (30 nmol/L), the cell length was decreased by 52.3±6.4% in normal group and 50.1±6.0% in IBS group. The response of muscle cells to [Sar9,Met(O2)11]-SP was not different between the two groups (Figures 2E-G).
Pretreatment of a NOS inhibitor L-NAME (0.1 mmol/L) increased the resting mean intraluminal pressure by 10.7±1.5% KCl (n = 7) in normal rat colon and by 12.9±3.4% KCl (n = 6) in IBS rat colon (P = 0.55). In normal rat colon (n = 7), the contractile effect of [Sar9,Met(O2)11]-SP was increased by the pretreatment of L-NAME to 17.2±2.2% KCl (P<0.01 vs 11.2±0.9% KCl), and to 22.1±1.5% KCl (P<0.01 vs 16.3±0.9% KCl) at 10 and 30 nmol/L, respectively. On the other hand, L-NAME was ineffective to augment the contractile effect of [Sar9,Met(O2)11]-SP in IBS rat colon (Figure 2H, 2I).
In the present study, we found that the NK1R-mediated colonic motor response was altered in a rat model of IBS. A selective NK1R agonist [Sar9,Met(O2)11]-SP contracted IBS rat colon more potently than normal rat colon. Because SP could stimulate both intestinal smooth muscle cells and enteric inhibitory nerves[10], we hypothesized that the higher contractile sensitivity of IBS rat colon to the NK1R agonist resulted from increased contractile response of muscle cells, and/or decreased response of enteric inhibitory nerves to the NK1R agonist. Our results support the second hypothesis. In normal rat colon, the contractile effect of [Sar9,Met(O2)11]-SP was enhanced by a neurotransmission blocker TTX, whereas that in IBS rat colon was not significantly changed by TTX. Furthermore, the [Sar9,Met(O2)11]-SP-induced contraction was not different between the two groups when the agonist was challenged to the TTX-treated isolated colon or directly to the isolated myocytes. These data indicate that the higher contractile sensitivity of IBS rat colon to [Sar9,Met(O2)11]-SP results from the decreased enteric inhibitory neural components rather than the increased contractile response of muscle cells.
Recently, enteric nitrergic inhibitory nerves were reported to participate in the NK1R-mediated control of peristalsis in isolated guinea-pig ileum[18] and in isolated rabbit distal colon[19]. Therefore, we supposed that nitrergic inhibitory nerves were the inhibitory neural components activated by [Sar9,Met(O2)11]-SP. Expectedly, we found that the [Sar9,Met(O2)11]-SP-induced contraction was augmented by the suppression of nitrergic inhibitory transmission with L-NAME in normal rat colon but not in IBS rat colon. Putting these lines of evidence together, it can be concluded that the increased NK1R-mediated contraction in IBS rat colon results from the decreased NK1R-mediated activation of enteric nitrergic inhibitory nerves.
Considering that the IBS rats used in this study developed IBS symptoms after subsidence of colitis, it is worthy to mention that intestinal inflammation could induce profound changes in enteric nerves, which might persist long after the inflammation subsided[20,21]. In addition, there have been studies reporting the dysfunction of enteric nitrergic nerves in animals with gut inflammation. Researchers have shown the decreased nNOS-immunoreactivities in TNBS-induced colitic rats[22], the reduced activity and synthesis of nNOS in DSS-induced colitic rats[23], and the diminished NO-mediated relaxation in nematode-infected mice[24]. Thus, it seems likely that alterations of enteric nitrergic neural function by colitis can persist in the colon of IBS rats, causing a higher contractile sensitivity of IBS colon to the NK1R agonist.
Since nitrergic nerves are tonically active in rat colon[25,26], one would expect that dysfunction of enteric nitrergic nerves results in alterations of the resting colonic motility. However, we observed that the resting motility of the isolated colon was not different between normal group and IBS group (Figure 1). Moreover, the extent of the increase in the resting colonic motor activities by a NOS inhibitor was similar in the two groups, suggesting that the tonic nitrergic inhibition of resting motility is maintained in IBS rat colon. This seems incompatible with our aforementioned conclusion that the decreased enteric nitrergic inhibitory neural components in IBS rat colon causes the increased NK1R-mediated colonic motor response. One of the possible explanations for this discrepancy is that the NK1R-mediated signaling pathways do not modulate the tonic inhibitory action of nitrergic nerves and hence have no influence on the resting colonic motility. Supporting this notion, Mule et al[27] reported that the resting spontaneous motility of rat colon was not affected by a NK1R antagonist but inhibited by several NK2R antagonists, indicating that the NK1R-mediated signaling pathways do not contribute to the control of the resting motility in rat colon.
In conclusion, the present results indicate that the NK1R-mediated contraction is exaggerated in the colon of rat IBS model. The higher contractile sensitivity of IBS rat colon to the NK1R agonist appears to result from the decreased enteric nitrergic inhibitory neural components rather than the increased contractile response of muscle cells. These results suggest that disordered defecation in IBS patients, especially who develop IBS after intestinal inflammation, might be related to the alterations in the NK1R-mediated control of bowel motility.
Edited by Wang XL
| 1. | Camilleri M, Heading RC, Thompson WG. Clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Drossman DA. Review article: an integrated approach to the irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13 Suppl 2:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol. 2002;35:S11-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Clemens CH, Samsom M, Van Berge Henegouwen GP, Smout AJ. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Chaudhary NA, Truelove SC. Human colonic motility. A comparative study of normal subjects, patients with ulcerative colitis, and patients with the irritable colon syndrome. Gastroenterology. 1968;54:Suppl: 777-uppl: 778. [PubMed] |
| 6. | Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Connell AM. Intestinal motility and the irritable bowel. Postgrad Med J. 1984;60:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Vassallo MJ, Camilleri M, Phillips SF, Steadman CJ, Talley NJ, Hanson RB, Haddad AC. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73:173-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 235] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Scheurer U, Drack E, Halter F. Substance P activates rat colonic motility via excitatory and inhibitory neural pathways and direct action on muscles. J Pharmacol Exp Ther. 1994;271:7-13. [PubMed] |
| 11. | Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884-G892. [PubMed] |
| 12. | Di Sebastiano P, Grossi L, Di Mola FF, Angelucci D, Friess H, Marzio L, Innocenti P, Büchler MW. SR140333, a substance P receptor antagonist, influences morphological and motor changes in rat experimental colitis. Dig Dis Sci. 1999;44:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ikeda K, Miyata K, Orita A, Kubota H, Yamada T, Tomioka K. RP67580, a neurokinin1 receptor antagonist, decreased restraint stress-induced defecation in rat. Neurosci Lett. 1995;198:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Okano S, Nagaya H, Ikeura Y, Natsugari H, Inatomi N. Effects of TAK-637, a novel neurokinin-1 receptor antagonist, on colonic function in vivo. J Pharmacol Exp Ther. 2001;298:559-564. [PubMed] |
| 15. | La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791-2795. [PubMed] |
| 16. | Coupar IM, Liu L. A simple method for measuring the effects of drugs on intestinal longitudinal and circular muscle. J Pharmacol Toxicol Methods. 1996;36:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982;395:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 707] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 18. | Holzer P. Involvement of nitric oxide in the substance P-induced inhibition of intestinal peristalsis. Neuroreport. 1997;8:2857-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Onori L, Aggio A, Taddei G, Loreto MF, Ciccocioppo R, Vicini R, Tonini M. Peristalsis regulation by tachykinin NK1 receptors in the rabbit isolated distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;285:G325-G331. [PubMed] |
| 20. | Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Miampamba M, Sharkey KA. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol Motil. 1999;11:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Mizuta Y, Isomoto H, Takahashi T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium. Gastroenterology. 2000;118:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Middleton SJ, Cuthbert AW, Shorthouse M, Hunter JO. Nitric oxide affects mammalian distal colonic smooth muscle by tonic neural inhibition. Br J Pharmacol. 1993;108:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Mulè F, D'Angelo S, Amato A, Contino I, Serio R. Modulation by nitric oxide of spontaneous mechanical activity in rat proximal colon. J Auton Pharmacol. 1999;19:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Mulè F, D'Angelo S, Tabacchi G, Serio R. Involvement of tachykinin NK2 receptors in the modulation of spontaneous motility in rat proximal colon. Neurogastroenterol Motil. 2000;12:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |