Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.212
Revised: February 5, 2004
Accepted: April 5, 2004
Published online: January 14, 2005
AIM: To investigate the adhesive mechanical properties of different cell cycle human hepatoma cells (SMMC-7721) to human umbilical vein endothelial cells (ECV-304), expression of adhesive molecule integrinβ1 in SMMC-7721 cells and its contribution to this adhesive course.
METHODS: Adhesive force of SMMC-7721 cells to endothelial cells was measured using micropipette aspiration technique. Synchronous G1 and S phase SMMC-7721 cells were achieved by thymine-2-deoxyriboside and colchicines sequential blockage method and double thymine-2-deoxyriboside blockage method, respectively. Synchronous rates of SMMC-7721 cells and expression of integrinβ1 in SMMC-7721 cells were detected by flow cytometer.
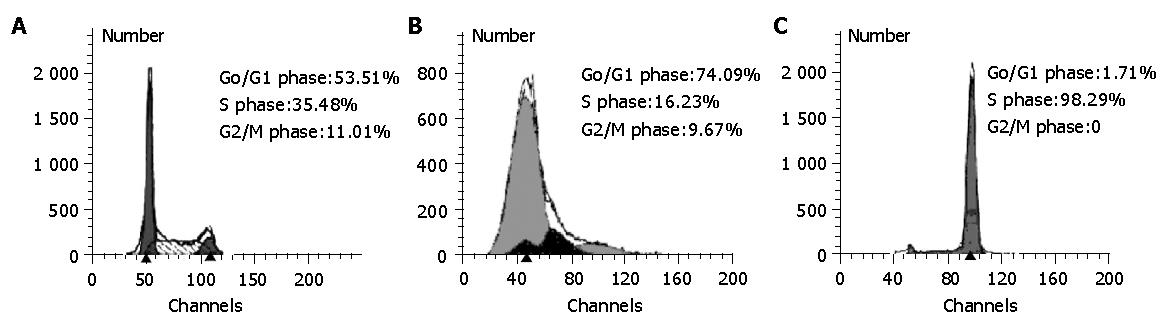
RESULTS: The percentage of cell cycle phases of general SMMC-7721 cells was 11.01% in G2/M phases, 53.51% in G0/G1 phase, and 35.48% in S phase. The synchronous rates of G1 and S phase SMMC-7721 cells amounted to 74.09% and 98.29%, respectively. The adhesive force of SMMC-7721 cells to endothelial cells changed with the variations of adhesive time and presented behavior characteristics of adhesion and de-adhesion. S phase SMMC-7721 cells had higher adhesive forces than G1 phase cells [(307.65±92.10)×10-10N vs (195.42±60.72)×10-10N, P<0.01]. The expressive fluorescent intensity of integrinβ1 in G1 phase SMMC-7721 cells was depressed more significantly than the values of S phase and general SMMC-7721cells. The contribution of adhesive integrinβ1 was about 53% in this adhesive course.
CONCLUSION: SMMC-7721 cells can be synchronized preferably in G1 and S phases with thymine-2-deoxyriboside and colchicines. The adhesive molecule integrinβ1 expresses a high level in SMMC-7721 cells and shows differences in various cell cycles, suggesting integrin β1 plays an important role in adhesion to endothelial cells. The change of adhesive forces in different cell cycle SMMC-7721 cells indicates that S phase cells play predominant roles possibly while they interact with endothelial cells.
- Citation: Song GB, Qin J, Luo Q, Shen XD, Yan RB, Cai SX. Adhesion of different cell cycle human hepatoma cells to endothelial cells and roles of integrin β1. World J Gastroenterol 2005; 11(2): 212-215
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/212.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.212
Tumor is a malignant disease caused by disorganized cell cycle and the uncontrollable growth of cells, which is very harmful to human’s health. Invasion and metastasis are the representations of malignancy and also the direct causes of terminal deterioration and death of tumor patients[1,2]. The metastasis of tumor cells through blood circulation is a complicated continuous process with many steps, of which the adhesion of tumor cells to extra cellular matrix (ECM) and endothelial cells is one of the definitive steps in tumor metastasis, and the expression of adhesive molecules is the important factor in determining the adhesion of cells. Integrinβ1 is the main mediating adhesive molecule in locking phase of the adhesion between tumor cells and vascular endothelial cells[3].
Primary hepatocellular carcinoma is one of the ten important frequent cancers in the world, but its biomechanical mechanism in the course of invasion and metastasis has not been elucidated completely up to now, the adhesive mechanical properties of different cell cycle hepatoma cells to endothelial cells and the expressions and roles of correlative adhesive molecules in the adhesive course have not been fully understood. In this paper, we put emphasis on studying the adhesive mechanical properties of different cell cycle hepatoma cells to endothelial cells using micropipette aspiration technique from the view of cell cycle. Furthermore, the expression and contribution of adhesive molecule integrinβ1 were investigated quantitatively. The experimental results may provide the quantitative biomechanical basis in understanding the metastasis mechanism of hepatoma cells.
Human hepatoma SMMC-7721 cells, purchased from the Second Military Medical University in Shanghai, were cultivated in RPMI-1640 medium (Gibco product) supplemented with 10% calf serum in a standard incubator at 37 °C.
Human umbilical vein endothelial ECV-304 cells, purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, were cultivated in M199 medium (Gibco product) supplemented with 15% fetal bovine serum (FBS, Hyclone product). ECV-304 cells were identified as endothelial cells by the presence of factor VIII-related antigen and typical endothelial morphology. Experiments were performed by using ECV-304 cells from passages 3-5.
G1 phase SMMC-7721 cells were achieved by thymine-2-deoxyriboside (Sigma-Aldrich product) and colchicine (Serva product) sequential blockage method. S phase SMMC-7721 cells were achieved by double thymine-2-deoxyriboside blockage method[4,5]. The synchronized SMMC-7721 cells were washed twice in 0.01 mol/L pH7.4 phosphate buffered solution (PBS), and fixed with 75% cooled ethanol, dyed for 30 min by propidium iodide (PI), then the synchronizing rate was measured by flow cytometer (BD FACS Caliber, BD Bioscience, USA).
About 1.5 mL cell suspension (1×106 cells/mL) was put into a 2.0-mL test tube and centrifuged at 1 500 rpm for 5 min. The cells were washed twice in 0.01 mol/L pH7.4 PBS, the supernatant was swilled out and about 200 μL solution was left, the solution was mixed gently and put into two 1.5-mL test tubes averagely. Then, 2.5 μL monoclonal mouse anti-human IgG1k/FITC (Ancell product, USA) was added into one test tube (as control) and 2.5 μL monoclonal mouse anti-human integrin β1/FITC (Ancell product, USA) was added into the other tube, they were let to react at 4 °C for 30 min. The cells were washed three times as before and the expression of integrin β1 was detected by a flow cytometer (BD FACS Caliber, BD Bioscience, USA).
Micropipette aspiration technique[6-9] was adopted to measure the adhesive force of SMMC-7721 cells to ECV-304 cells. Briefly, monolayer ECV-304 cells were cultivated in a special chamber, about 0.5 mL SMMC-7721 single cell suspension (1×105 cells/mL) was added into the chamber, adhesive experiments were started about 30 min later. The pressure was adjusted to zero (zero-pressure state) using the pressure controlling and recording system. SMMC-7721 cells were chosen under a microscope and the tip of the micropipettes was positioned close to the surface of SMMC-7721 cells by a micromanipulator, a small portion of the cells was aspirated into the micropipettes with a step-rise negative pressure produced by the pressure control system. Then the micropipettes were pulled using the micromanipulator flatly and slowly, SMMC-7721 cells were detached gradually from the surface of adhered ECV-304 cells, and the negative pressure was adjusted simultaneously until the SMMC-7721 cells were drawn out from the surface of adhered ECV-304 cells. The whole experimental process was recorded using a video tape recorder continuously, the experimental data were measured through the image processing system. All micropipette manipulations were carried out at room temperature (about 25 °C) and completed within 2 h.
The formula for calculating the adhesive force (F) was defined as[10,11], F = p×R2×ΔP×cos q, where Rp is the inner radius of the micropipettes (2.5-3.0 μm inner radius was used in this experiment),ΔP is the critical negative pressure, and q is the angle between the micropipettes and the plane of ECV-304. In our experiments, q angle was regulated to about 10o, therefore cos q ≈ cos10o = 0.985 ≈ 1 and the formula could be simplified as F = p×R2×ΔP
The adhesive force values were described as mean±SD and Student’s t test was used for statistical analysis.
Cell cycle phases were analyzed with a flow cytometer. The percentage of cell cycle phase of general SMMC-7721 cells (non-synchronized) was 53.51% in G0/G1 phase, 11.01% in G2/M phases, 35.48% in S phase, as shown in Figure 1A. On the other hand, we synchronized SMMC-7721 cells by the methods of thymine-2-deoxyriboside and colchicines sequential blockage and double thymine-2-deoxyriboside blockage, the average synchronizing rates of G1 and S phase cells amounted to 74.09% and 98.29%, respectively (Figure 1B, 1C). It was suggested that SMMC-7721 cells could be synchronized preferably in G1 and S phases using this method.
Using the micropipette aspiration technique, we investigated the time dependency of adhesive forces of SMMC-7721 cells to ECV-304, the results are shown in Table 1.
| Period of time (min) | Adhesion forces (F×10-10N) | n |
| 0-30 | 39.98±25.77 | 16 |
| 30-60 | 297.32±82.35 | 20 |
| 60-90 | 336.49±73.51 | 18 |
| 90-120 | 301.09±62.04 | 21 |
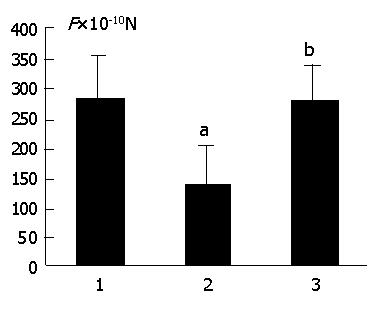
The adhesive forces of different cell cycle SMMC-7721 cells to ECV-304 cells were investigated, and the results are shown in Table 2. The adhesive forces of G1 phase SMMC-7721 cells to ECV-304 cells were much lower than the corresponding value of S phase (P<0.01) and general SMMC-7721 cells (P<0.01). However, there was no obvious difference in the adhesive forces between S phase and general SMMC-7721 cells.
| Treatment | Adhesion forces (F×10-10N) | n |
| General SMMC-7721 | 281.26±70.15 | 38 |
| G1 phase SMMC-7721 | 195.42±60.72b,d | 31 |
| S phase SMMC-7721 | 307.65±92.10 | 35 |
The expressed fluorescent intensity of integrin β1 in different cell cycle SMMC-7721 cells is shown in Table 3, which indicates that the average fluorescent intensity of integrin β1 in general, G1 phase and S phase SMMC-7721 cells was 95.7±1.2, 76.9±1.9, and 94.1±1.6, respectively. It could also be known from the table that the expression of integrin β1 in G1 phase was decreased obviously when compared with the corresponding value of S phase and general SMMC-7721 cells. These results suggested that there were cell cycle differences of expressions of integrin β1 in SMMC-7721 cells.
| Treatments | Expressed fluorescent intensity of integrin β1 | n |
| General SMMC-7721 | 95.7±1.2 | 3 |
| G1 phase SMMC-7721 | 76.9±1.9b,d | 3 |
| S phase SMMC-7721 | 94.1±1.6 | 3 |
To study the contributions of integrin β1 to the adhesion of hepatoma cells to endothelial cells, we measured the adhesive forces quantitatively of general SMMC-7721 cells to ECV-304 cells with micropipette aspiration technique, using monoclonal mouse anti-human IgG1k/FITC (nonspecific antibody) and monoclonal mouse anti-human integrin β1/FITC (specific antibody) to block these adhesive courses, respectively. The results are shown in Figure 2. The adhesive behavior of SMMC-7721 cells to ECV-304 cells was obviously blocked by monoclonal antibody of integrin β1, the block rate was 53.01% and the adhesive force was distinctly lower than that of general SMMC-7721(P<0.01). However, the block rate of nonspecific antibody was only 1.52% and the adhesive force had no difference when compared with control experiment.
Adhesion of tumor cells to vascular endothelial cells is one of the key steps in metastasis. It has been proved that there is much commonness in the course of interactions between tumor cells and different vascular endothelial cells despite of organic metastasis[12]. Now adhesive behaviors of tumor cells to human umbilical vein endothelial cells have been used as a “gold model” for studying interactions of tumor cells and endothelial cells[13], and this model has been accepted by academic communities[14]. In this paper, we explored the adhesive mechanical properties of human hepatoma cells and human umbilical vein endothelial cells from the view of cell cycle and found that the adhesive forces of SMMC-7721 cells to ECV-304 cells changed with adhesive time, and increased rapidly within 30-60 min and then kept steady after 60 min. This suggests that the expression of adhesive molecules is time-dependent in SMMC-7721 cells and ECV-304 cells, thas showing behavior characteristics of adhesion and de-adhesion.
With a series of ordinary biochemical events in space-time, cells could control their growth, proliferation, differentiation to ensure the natural process of cell cycle[15]. The abnormity of cell cycle regulating function is one of the mechanisms that result in the abnormal proliferation of tumor cells. Therefore it is very important to understand the proliferation, differentiation and the biophysical characteristics of cells from the view of cell cycle, while the cellular synchronization is the important way to investigate cell modalities, structures, functions and other characteristics in each cycle phase. The synchronous results have shown that thymine-2-deoxyriboside and colchicine sequential blockage and double thymine-2-deoxyriboside blockage methods could synchronize SMMC-7721 cells preferably in G1 phase and S phase, respectively. Comparing the synchronous results, we found that the synchronizing rate of G1 phase SMMC-7721 cells was lower than that of S phase, which might be caused by different synchronizing ways.
To some extent, the occurrence, evolution and metastasis of malignant tumors are related to the abnormal expression or structural change of integrin[16]. There is a decrease or lack of the integrin expression in some tumor cells while a distinct increase in other tumors[17]. This indicates that the relation between integrin and tumors might be twofold. In the early period of tumor occurrence, the decrease of expression of integrin might result in the weakening of adhesion between tumor cells and basement membranes or extra cellular matrix, which is proportional to the local growth of tumor cells. After tumor cells enter blood circulation, increased expression of integrin would be proportional for tumor cells to adhere to vascular endothelial cells and its metastasis. Integrin β1 is the main mediating adhesive molecule in locking phase of adhesions between tumor cells and vascular endothelial cells. In this experiment, we found that strong fluorescence of expressed integrin β1 occurred in SMMC-7721 cells, but the expression of integrin β1 in G1 phase SMMC-7721 cells was decreased obviously when compared with the corresponding values of S phase and general SMMC-7721 cells, there were cell cycle differences in expression of integrin β1 in SMMC-7721 cells.
In our study, we found that the adhesive force was obviously decreased after the blockage using the specific monoclonal antibody of mouse integrin β1 (P<0.01), the block rate was 53%. Meanwhile blocked by nonspecific monoclonal antibody under the same condition, the adhesive force was almost equal to that of the control experiment and the block rate was only 2%. These results indicate that the expression of integrin β1 plays very important roles in the adhesion of SMMC-7721 cells to ECV-304 cells, and the contribution of this adhesive molecule is about 50% in these adhesive processes.
We also discovered that the adhesive force of G1 phase SMMC-7721 cells was distinctly lower than the corresponding value of S phase cells (P<0.01), but there was no obvious difference when S phase was compared with general SMMC-7721 cells. This result suggests that S phase SMMC-7721 cells probably play a very important role in the adhesive course between SMMC-7721 cells and ECV-304 cells. Compared to the results we achieved in the investigation of the adhesive force of different cell cycle SMMC-7721 cells to surfaces coated with collagen IV[18], we conclude that G1 phase SMMC-7721 cells might be very important in promoting the interaction with basement membranes in the course of metastasis through blood circulation. However, S phase SMMC-7721 cells might play predominant roles in the adhesion of SMMC-7721 cells to ECV-304 cells.
Invasion and metastasis of hepatoma cells are a complicated process, the adhesive behaviors of hepatoma cells are mediated by the cooperation of many adhesive molecules. The functions, contributions and rules of other adhesive molecules in these adhesive courses should be further studied.
We would thank Professor Long Mian (Institute of Mechanics, The Chinese Academy of Sciences) for his advise in this experiment.
Assistant Editor Guo SY Edited by Wang XL and Ma JY
| 1. | Thiery JP. Cell adhesion in cancer. Comptes Rendus Physigue. 2003;4:289-304. [DOI] [Full Text] |
| 2. | Jiang XN, Zhou RL. The Relatiosnhip Between Adhesion,Migration of Cancer Cellsand Mestastasis. Shengwu Huaxue Yu Shengwu Wuli Jinzhan. 1998;25:404-407. |
| 3. | Karmakar S, Mukherjee R. Integrin receptors and ECM proteins involved in preferential adhesion of colon carcinoma cells to lung cells. Cancer Lett. 2003;196:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Uzbekov R, Chartrain I, Philippe M, Arlot-Bonnemains Y. Cell cycle analysis and synchronization of the Xenopus cell line XL2. Exp Cell Res. 1998;242:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Song GB, Yu WQ, Liu BA, Long M, Wu ZZ, Wang BC, Cai SX. Investigation on the viscoelasticity of synchronous human hepatocellular carcinoma cells. Colloids and Surfaces B: Biointerfaces. 2002;24:327-332. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Geiger S, Jager-Lezer N, Tokgoz S, Seiller M, Grossiord JL. Characterization of the mechanical properties of a water/oil/water multiple emulsion oily membrane by a micropipette aspiration technique. Colloids and surfaces A: Physicochemical and Engineering Aspects. 1999;157:325-332. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | You J, Mastro AM, Dong C. Application of the dual-micropipet technique to the measurement of tumor cell locomotion. Exp Cell Res. 1999;248:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Zhao H, Dong X, Wang X, Li X, Zhuang F, Stoltz JF, Lou J. Studies on single-cell adhesion probability between lymphocytes and endothelial cells with micropipette technique. Microvasc Res. 2002;63:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Wu ZZ, Wang BC, Shao KF, Akio S. Adhesion of normal and carcinoma hepatic cells on the membrane containing collagen IV using a micropipette technique. Colloids and Surfaces B: Biointerfaces. 1998;11:273-279. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Wu ZZ, Shao KF, Song GB, Wang HB, Wang YJ, Cai SX. Adhesion of hepatocellular carcinoma cells to collagen IV coated surfaces. Natl Med J China. 1999;79:369-372. |
| 11. | Song GB, Liu BA, Qin J, Long M, Cai SX. Studies on the adhesive mechanical properties between hepatocellular carcinoma cells and endothelial cells. Shengwu Wuli Xuebao. 2003;19:84-87. |
| 12. | Weber GF, Ashkar S. Molecular mechanisms of tumor dissemination in primary and metastatic brain cancers. Brain Res Bull. 2000;53:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Simiantonaki N, Jayasinghe C, Kirkpatrick CJ. Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro. Exp Mol Pathol. 2002;73:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res. 2001;97:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Crowe DL, Brown TN, Kim R, Smith SM, Lee MK. A c-fos/Estrogen receptor fusion protein promotes cell cycle progression and proliferation of human cancer cell lines. Mol Cell Biol Res Commun. 2000;3:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Han JY, Kim HS, Lee SH, Park WS, Lee JY, Yoo NJ. Immunohistochemical expression of integrins and extracellular matrix proteins in non-small cell lung cancer: correlation with lymph node metastasis. Lung Cancer. 2003;41:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Armulik A, Svineng G, Wennerberg K, Fässler R, Johansson S. Expression of integrin subunit beta1B in integrin beta1-deficient GD25 cells does not interfere with alphaVbeta3 functions. Exp Cell Res. 2000;254:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Yu W, Song G, Long M. Investigation on the adhesive properties of different cycle hepatoma cells. Zhonghua GanZangBing ZaZhi. 1999;7:153-155. [PubMed] |