Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2960
Revised: June 29, 2004
Accepted: July 22, 2004
Published online: May 21, 2005
AIM: To obtain the short peptides mimic antigenic epitopes selected by rat natural antibodies to schistosomes, and to explore their immunoprotection against schistosomiasis in mice.
METHODS: Adults worm antigens (AWA) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and enzyme-linked transferred immunoblotting methods with normal SD rat sera (NRS). The killing effects on schistosomula with fresh and heat-inactivated sera from SD rats were observed. Then the purified IgG from sera of SD rats was used to biopan a phage random peptide library and 20 randomly selected positive clones were detected by ELISA and 2 of them were sequenced. Sixty female mice were immunized thrice with positive phage clones (0, 2nd, 4th wk). Each mouse was challenged with 40 cercariae, and all mice were killed 42 d after challenge. The worms and the liver eggs were counted.
RESULTS: NRS could specifically react to the molecules of 75000, 47000, 34500 and 23000 of AWA. Sera from SD rats showed that the mortality rate of schistosomula was 76.2%, and when the sera were heat-inactivated in vitro, the mortality rate was decreased to 41.0% after being cultured for 48 h. The specific phages bound to IgG were enriched about 300-folds after three rounds of biopanning. Twenty clones were detected by ELISA, 19 of them bound to the specific IgG of rat sera. Immunization with these epitopes was carried out in mice. Compared with the control groups, the mixture of two mimic peptides could induce 34.9% (P = 0.000) worm reduction and 67.6% (P = 0.000) total liver egg reduction in mice. Two different mimic peptides could respectively induce 31.0% (P = 0.001), 14.5% (P = 0.074) worm reduction and 61.2% (P = 0.000), 35.7% (P = 0.000) total liver egg reduction. The specific antibody could be induced by immunization of the mimic peptides, and the antibody titer in immunized mice reached more than 1:6400 as detected by ELISA.
CONCLUSION: Specific peptides mimic antigenic molecules can be obtained by biopanning the phage random peptide library and a partially protective immunity against schistosome infection can be stimulated by these phage epitopes in mice.
- Citation: Wang M, Yi XY, Li XP, Zhou DM, Larry M, Zeng XF. Phage displaying peptides mimic schistosoma antigenic epitopes selected by rat natural antibodies and protective immunity induced by their immunization in mice. World J Gastroenterol 2005; 11(19): 2960-2966
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2960.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2960
It is a major strategy to develop vaccines against schistosomiasis recommended by the World Health Organization[1]. In recent years, studies on vaccines have progressed rapidly, and a series of vaccine candidates have been identified and tested against schistosome infection in experimental models[2]. Nevertheless, these vaccines provide only 20-40% protection against the challenge of schistosoma cercariae. The protection so far achieved could not meet the need for control of this disease[3-5]. So scientists now intend to use new techniques for developing anti-schistosomiasis vaccines. Phage displaying technique appears to be a very powerful and promising technique.
Schistosome has a very broad range of hosts including more than 40 kinds of mammals, but adaptability and permissibility of the parasite in various mammals are different. Chinese researchers have discovered that the growth and development of schistosomes in rats are slower than those in permissive hosts. According to the research of Yu et al[6], the worms and egg-granulomas in rat livers were rarely observed when rats were challenged with Schistosoma japonicum (S.j) cercariae. The majority of eggs were unripe eggs and black dead eggs and the number of mean liver eggs per gram (LEPG) was 1393.46, apparently less than that in the permissive host mouse. These results indicate that some factors are related to the natural resistance that existed in rats. Our previous research[7] showed that the levels of IgG to adult worm antigens (AWA) and soluble egg antigens (SEA) in normal rat sera (NRS) and infected rat sera (IRS) were significantly higher than the corresponding levels observed in the uninfected mice sera. High levels of protection against schistosomes could be induced. Now the common understanding acknowledged is that rats are semi-permissive hosts of schistosomes that have been used as an experimental model for vaccine development.
The work described in this paper was performed to explore the immunological characteristics of natural resistance to S.j infection in rats, evaluate the killing effects on schistosomula in vitro, and determine whether individual peptides selected from a phage random peptide library of 12-mer by the NRS could induce the immune protection in mice. The results showed that the specific antibodies existed in rats, which play an important role in killing the larva, and the two peptides (P3, P5) selected from the library in this way can induce an antibody response, which confer a partial protection against infection in mice. These experimental data provide the basis for a peptide vaccine.
Phage library and host strain E.coli ER 2738 were a kind present from Professor Larry McReynolds in New England Biolabs, USA. The library, which was based on a combined library of random peptide 12-mer fused to a minor coat protein (pIII), consists of 2.7×109 electroporated sequences and the phage titer is 1.5×1012 pfu/mL.
All experimental animals were provided by the Animal Center, Central South University, Xiangya School of Medicine, including 10 SD female rats (300-350 g), 10 male mice (25-30 g) and 60 female mice (18-22 g) of Kunming strain and 4 male rabbits (1.5-2 kg).
S.j cercariae were released from Oncomelania hupensis purchased from Hunan Institute of Parasitic Diseases, YueYang, China.
All reagents and chemicals used in this study were of analytical grade or the best quality purchased from domestic and international companies.
The rats and 10 male mice were given 500 and 40 S.j cercariae respectively, killed 45 d after infection, and the infected sera (IRS and IMS) were collected. Normal rat sera (NRS) and normal mice sera (NMS) were obtained before challenge infection. Rabbit sera pool (RS) was taken and heat-inactivated NRS were incubated at 56 °C for 30 min in an attempt to deplete the complements. All sera were filtered with a microcell filter (Φ 0.22 μm) to eliminate RBC fragments and bacteria.
S.j AWA was separated by 10% sodium dodecylsulfate polyacrylamide gels (SDS-PAGE). After electrophoresis (20 mA for 2 h), the separated proteins were transferred onto a nitrocellulose membrane (120 mA for 2 h), and then the membrane was blocked with 3% non-fat milk. After that, nitrocellulose strips were incubated with 1:100 diluted NRS, IRS, NMS and IMS for 2 h at 37 °C respectively. The strips were cultured with horseradish peroxidase-labeled goat -anti-mouse IgG conjugates (1:3000 dilution) for 2 h after being washed. Following washing, the strips were visualized by staining with 3,3’-diaminobenzidine and the molecular weight of tested proteins was calculated according to RF value of the marker.
The cercariae released from Oncomelania hupensis were collected for 10 min on ice. After being spun for 5 min at 1 500 r/min, the supernatant was decanted. The pellet was washed and centrifuged thrice with Earle’s culture medium containing 300 U/mL penicillin and 300 μg/mL streptomycin. Then the cercariae were suspended in RPMI 1640 containing 35% heat-inactivated rabbit sera. The suspension was subsequently added to 24-well culture plates and the density was adjusted to 200±20 cercariae/mL and maintained at 37 °C in a 5% atmosphere in 1 mL of RPMI 1640 culture medium (100 U/mL penicillin, 100 μg/mL streptomycin).
Duplicate wells of a 24-well flat-bottomed microtiter plates were placed in RPMI 1640 containing NRS, heat-inactivated NRS, NMS and RS with a concentration of 10%. The number of dead and alive schistosomula was counted under a reverse microscope after in vitro culture for 24, 48 and 72 h. The mortality was calculated [the mortality (%) = (dead schistosomula/total schistosomula)×100%]. The judgment criteria of dead schistosomula were referred to Vadas et al[8]. The interior structure was vague, the tegument membrane was destroyed, and the inside substance was released as radiation, or the schistosomula was inactive with an obscure structure.
Serum pools were obtained from normal rats. The IgG from rat sera was purified by ammonium sulfate precipitation method (50-33-33%). Then the supernatant was dialyzed against phosphate-buffered saline (PBS, pH 7.2). Microtiter wells were coated overnight at 4 °C with 100 μg/well of the purified IgG from NRS. The plates were then blocked with 3% nonfat milk for 2 h at 37 °C, washed five times with 0.05% Tween-20 in Tris-buffered saline (PBST). One hundred microliters of the diluted library containing 1.5×1011 pfu was added to the coated plates and incubated at room temperature for 1 h. Following that, bound phages were subsequently eluted with 100 μL of 0.2 mol/L glycine-HCl (pH 2.2) and neutralized with 1 mol/L Tris-HCl (pH 9.1). Then the eluted phages were amplified in host strain E.coli 2738 and purified by precipitation for about 4 h using 1/6 volume of PEG/NaCl. Another two rounds of affinity selection were carried out in the same way, but 1:200 and 1:400 sera dilution was used and added to 100 μL of diluted phages from those of the last round. The percentage of enrichment of phage clones was calculated using the following formula: percentage enrichment of phage clones (%) = (eluted phages/added phages)×100%.
The eluted phages were added to the host strain ER2738 culture (A600-0.5) and incubated at 37 °C with vigorous shaking for 4.5 h. The culture was transferred to a tube and spun at 10000 g for 10 min at 4 °C. The supernatant was transferred and spun once again. Then the upper 80% of the supernatant was removed to a fresh tube and 1/6 volume of PEG/NaCl was added. Then the phages were allowed to precipitate at 4 °C overnight. The supernatant was spun and decanted at 12000 g for 10 min. The suspended deposit was precipitated with PEG again. After being incubated on ice for 1 h and spun for 20 min, the supernatant was discarded and the pellet was suspended in 200 μL TBS, 0.02% NaN3. After being micro-centrifuged for 1 min, remaining insoluble matter was pelleted, the supernatant contained the amplified phages. The amplified phages were diluted by a series of different titers. Ten microliters of each dilution was added to 200 μL host strain culture, mixed and incubated for 20 min at 37 °C. Then cells were transferred to a culture tube containing 45 °C agarose, quickly vortexed and immediately poured onto pre-warmed LB plates. After being cooled, the plates were inverted and incubated for 16 h at 37 °C. The plates were inspected and the plaques on plates were counted. The phage titer was obtained.
Ten microliters of eluted phages from the third round was added to 200 μL of the host strain cultured overnight, and incubated for 20 min at 37 °C to finish transfection. Then the transfected cells were transferred to a culture tube with agarose, quickly vortexed and immediately poured onto a pre-warmed LB plate. After being cooled for 5 min, the plates were inverted and incubated overnight at 37 °C. On the 2nd d, 20 clones were randomly picked. Each selected clone was amplified, purified and tittered according to the procedure described above. The ELISA wells were coated with 2×1011 phage particles and then blocked with 3% nonfat milk. Afterwards 100 μL of 1:200 diluted NRS was added and allowed to bind for 2 h at 37 °C. The wells were subsequently washed with PBS containing 0.05% Tween-20 (PBST). After goat anti-mouse IgG conjugated to HRP was added, specially bound antibodies were visualized by adding 3,3’5,5’-tetramethylbenzidine. The absorbance was determined at 595 nm. The original phage library was used as a negative control. Phage clones were considered positive when the A value was 2.1-fold higher than that of the negative control.
Two phage clones that showed A values higher than the others were precipitated with PEG/NaCl. Their single-strand DNA was prepared by phenol extraction. These two phages were automatically sequenced on the ABI PRISM 377 sequencer using the -28 g111 sequencing primer (5’-110 GTA TGG GAT TTT GCT AAA CAA C-3’) from the phage display peptide library kit.
Female Kunming mice were divided into five groups. The experimental groups (P5+P3 mixture group, P5 group, P3 group) were injected with 0.2 mL positive clones (containing 1013 phage particles) thrice (0, 2nd, 4th wk). Two control groups were injected with original phages or TBS buffer. Blood samples were collected by tail vein puncture before immunization and challenge. The antibody response induced was analyzed by ELISA. At the 5th wk, each mouse was percutaneously infected with 40±1 S.j. cercariae. All mice were killed and perfused 45 d post-infection, and the worm and liver eggs were counted.
Significance testing was conducted by analysis of variance between experimental and control groups using one-way ANOVA of SPSS software. P<0.05 was considered statistically significant. Results were presented as mean±SD.
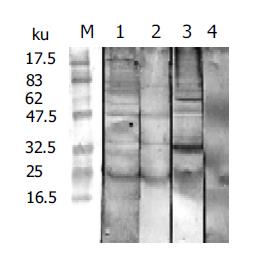
NRS was tested for its recognition of AWA by Western blot (Figure 1). The results showed that NRS and IRS could recognize four bands of AWA. Their molecular weight was 75000, 47000, 34500 and 23000, respectively.
Killing effects of different sera (NRS, heat-inactive NRS, NMS and RS) on schistosomula in vitro were studied in 24-, 48- and 72-h culture (Table 1). The mortality of larva after being cultivated for 48 and 72 h was not significantly different in each sera group. The mortality rates were 23% and 76.2% in NRS after 24- and 48-h cultivation, which showed that the killing effect of NRS was higher than those of other sera. Nevertheless, when the NRS was heated at 56 °C for 30 min to inactivate the complement components, the mortality rates were 16.9% and 41.0%, respectively. The result implied that the complements and the antibodies of NRS had significant effects on killing the early phase schistosomula in vitro.
| Sera | Schistosomula (n) | Mortality (%) | ||
| 24 h | 48 h | 72 h | ||
| NRS | 206 | 23.0 | 76.2 | 86.4 |
| Heat-inactive normal | 234 | 16.9 | 41.0 | 43.6 |
| rat sera | ||||
| NMS | 240 | 6.4 | 23.8 | 30.0 |
| RS | 231 | 4.7 | 16.9 | 21.2 |
The percentages of phage enrichment after three rounds of biopanning are presented in Table 2. In the condition of decreasing IgG antibody concentration gradually, the specific phages bound to IgG were increased. The eluted phages increased from 1.65×10-6 to 5×10-4, an enrichment of about 300-folds. The data reflected a successful affinity selection of phages that were ligands for particular antibodies.
| Biopanning | Phages added | Phages washed | Yield (%) |
| 1 | 1.5×1011 | 2.47×105 | 1.65×106 |
| 2 | 1.0×1011 | 4.26×106 | 4.26×105 |
| 3 | 1.0×1011 | 5.00×107 | 5.00×104 |
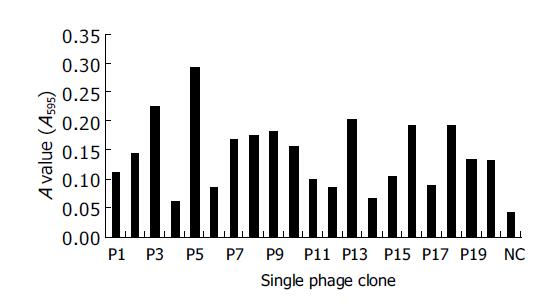
Twenty randomly selected individual clones were tested for their binding to rat sera by ELISA. Original phages were used as negative controls. The results showed that natural antibodies from NRS recognized 19 randomly selected clones. The positive percentage was 95% (Figure 2).
The single-strand DNA from two positive phages named P3 and P5 was sequenced. The DNA sequences and the deduced amino acid (AA) sequences are listed in Table 3. We have fed the sequence data on the Internet and analyzed them using nucleotide blast software of NCBI. The results revealed that there was no significant similarity with other sequences (score <50).
| Clone | DNA sequence | AA sequence |
| P3 | AAACGGAGGCTGCGGCCCAGT | K R R L R P S R V R I E |
| AGAGTGAGAATAGAA | ||
| P5 | AATATGCCTATTAGCATGAAG | N M P A S M K R V R I E |
| AGAGTGAGAATAGAA |
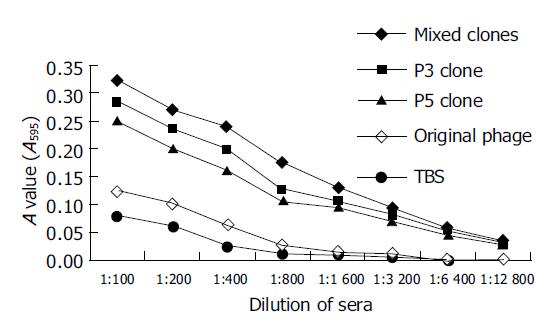
Compared with control groups, the specific antibodies could be stimulated and detected by ELISA in mice after the third immunization with positive phage clones, and the titer of specific antibodies was >1:6400, significantly higher than that in control groups (Figure 3).
All mice were perfused and the number of worms and the number of liver eggs were counted 42 d after being challenged with 40±1 S.j cercariae. Compared with the control groups, the group immunized with the mixture of two mimic peptides (P3+P5) showed 34.9% worm reduction and 67.6% total liver egg reduction. Two different groups immunized with P3 or P5 showed 31.0%, 14.5% worm reduction and 61.2%, 35.7% total liver egg reduction, respectively. Some immune protection could be seen in original phage controls, but there was no statistical significance between the two control groups (Tables 4 and 5).
Schistosomiasis is a serious parasitic disease with over 200 million people infected worldwide and causes about estimated 1 million deaths per year. In the past 50 years, though some control strategies have been successfully employed in some regions, the disease remains endemic in 76 developing countries and more than 600 million people are at risk of schistosoma infection. As investigated, the patients presented with a series of serious symptoms. Praziquantel, as a major drug for schistosomiasis, has been reported to develop resistance[9-11]. Furthermore, re-infection occurs rapidly in endemic regions. So it is the most effective strategy to develop vaccine against schistosomiasis. Scientists have studied different types of schistosomiasis vaccines. Despite the progress achieved, a feasible anti-schistosomiasis vaccine for humans or livestocks has not been found.
In contrast to mouse as a permissive host of schistosomes, rats could eliminate the primary schistosomes around 4 wk post-infection and subsequently developed protective immunity to re-infection. In rats with schistosomiasis, a predominant expression of Th2-type cytokine response at the mRNA level was shown after infection[12]. The protective immune mechanism was related to a Th2 response[12-14]. On d 4 and 7 post-infection, the morphology and size of schistosomula in the lung of rats were similar to those in mice, but there were strong inflammatory reactions around the schistosomula in the lung of rats. The inflammatory cells including eosinophils, neutrophils, macrophages and lymphocytes gathered around the larva and different levels of damage could be found on teguments. The inflammatory reactions in the lung of rats were significantly ameliorated on d 14 post-infection than before. These findings indicated that the immune response reacted mainly to the early-stage schistosomula[12,15]. In this model, several other studies have revealed that the resistance is due to antibody-dependent cell-mediated cytotoxicity mechanism that plays an essential role in destructing the young schistosomula in rats[16,17]. Passive transfer of sera from rats vaccinated with attenuated schistosoma cercariae conferred high levels of resistance against challenge. The worm reduction rate was up to 64%. Heat inactivation of sera at 56 °C for 3 h to deplete the IgE and complement components weakened the protective capacity[12].
The specific IgG to AWA and SEA was increased after infection in rats[6,7]. Passive transfer of NRS to mice, a reduction in worm burden and LEPG in transfer group were 19.1% and 46.4% compared with the control group[7]. In the present study, we observed that the NRS could specifically react to four bands with molecular weights of AWA, which were 75000, 47000, 34500 and 23000. The killing effects of NRS were accessed. The mortality was 23% and 76.2% in NRS after 24- and 48-h cultivation, higher than those of other sera. Nevertheless, when the complement was depleted, the mortality decreased to 16.9% and 41.0%, respectively. These data showed that the natural antibodies to schistosomes existed in rats and might play an important role in killing young schistosomula.
Phage display library technique has become a new powerful tool. Filamentous bacteriophages display foreign peptides on their surfaces. The technique involves specific screening and affinity selection of certain phage displaying peptides that are ligands for a particular protein, and the selected phages could be applied to affinity from 103- to 108-folds according to the process of absorption, elution and amplification. Furthermore it was easy to identify the DNA sequences of specific phage peptides selected[18]. Screening phage library could identify protein antigen epitopes[19], study protein interaction[20], search novel vaccines[21] and develop drugs[22]. Now the technique has been widely used in many fields including bacteria[23], viruses[24] and helminthes[25]. For instance, Jolive-Reynaud et al[26], screened a dodecapeptide library on phages with anti-NS3 mouse mAbs and human anti-HCV NS3 positive sera. Two peptides were selected since they were recognized specifically by the anti-NS3 mAbs and human sera from HCV-infected patients. Homology search showed that one shared AA similar to NS3 at residues 1396-1398 and the other had two AAs similar to nearby residues 1376 and 1378. Reproduced as synthetic dodecapeptides, the two mimotopes were recognized specifically by 12 and 22, respectively, out of 49 sera from HCV-infected patients. Other characteristics of the phages screened were that they could induce protection without the addition of any adjuvant. Because filamentous phages had the capability of activating CD4+ helper T cells[27], the phages were the ideal carrier of vaccines.
Due to the above characteristics, the technique has been used to develop vaccines against schistosomes in recent years. Arnon et al[28], screened a phase 8-mer random peptide library with the protective mAb against Schistosoma mansoni (152-66-9B). Four epitopes were obtained. Subsequently synthetic relevant peptides (P28-P31) were prepared. The peptides except P31 were recognized by 152-66-9B. Sera from mice immunized with the peptides were tested by ELISA. The results showed that each was recognized by the respective homologous anti-sera as well as by sera from infected mice with comparable titers. Moreover, worm burden was reduced by an average of 42±3% as a result of immunization with the P30 in mice. Fu et al[29], used the purified polyclonal IgG against the S.j 22.6 ku antigen to screen a 12-mer phage library. The polyclonal antibody could recognize 16 clones that were randomly picked. The sera from mice immunized with six clones of them were proved to have biological activities by Western blot. Six clones were sequenced, four epitopes were then identified. One epitope (H2) had three AA (IIL) sequences homologous to those of 22.6 ku. The mice immunized with the mixture of four peptide clones induced a significant worm reduction (39.5%) and total liver egg reduction (57.1%). Our previous studies also showed a protective immunity against schistosomiasis stimulated by mixed phage peptides in mice[30,31].
The work described in this paper was designed to explore whether or not the mimic peptides recognized by rat natural antibodies could induce protective immunity against S.j. It should be known that B-cell response was particularly important in the course of schistosomiasis and has been used to identify potential vaccine candidates[29,31]. Thus, the characteristics of natural antibodies in rats were tested. The results implied that natural antibodies which existed in rats had significant killing effects on schistosomula in vitro and the complement participated in the killing effects. A 12-mer phage random library was screened with the antibodies. It was hoped to determine that the mimic peptides could be identified in this way, and used to induce protective antibody response. Of the 20 randomly picked phage clones from a peptide library, 19 clones were shown to react specially with the NRS in ELISA and 2 clones (P3, P5) of them were bound at a higher level than the others. The two clones were analyzed to have partial homologous sequences (RVRIE). These data supported the views that these phages were able to mimic the binding properties of antigen epitopes. In a further study of the two clones, the mixed phages (P3+P5) and two individual phages (P3, P5) were used to immunize mice. Sera from mice immunized with phages had a higher binding than those immunized with the original peptides and TBS. The fact that high titers of antibodies were generated suggested that the immunization with these phages could result in the induction of protective responses. To test the phages for their protective effects, challenge experiment was performed. Significant protection was observed following immunization with the mixed phages that could induce 34.9% worm reduction and 67.6% total liver egg reduction in mice. This level of protection is similar to that of immunization with only P3 that induced 31.0% worm reduction and 61.2% total liver egg reduction, while the mice immunized with P5 induced only 14.5% worm reduction and 35.7% egg reduction. The results showed partial protection against challenge infection. Though the liver egg reduction rate that was 18.0% (P = 0.013) in original phage control group had a statistical significant compared with TBS control group, there was no actual significance in experiments. As far as the protective mechanism was concerned, the antibody in mice was tested. The titers of specific antibodies in mice immunized with phages were significantly higher than those in control groups (Figure 3). But there was an insignificant difference between the two groups immunized with individual phages P3 and P5. Because the antibody isotypes or cytokine expression was not detected, we could not better understand the protection.
Furthermore, though the two peptides had the core sequence, the protection induced was different. Whether the core sequence or the sequence alongside plays an important role has to be further studied. In a word, the results obtained demonstrated that the protective immunity could be induced by the selected phages without adjuvant.
In conclusion, natural antibodies to S.j that existed in rats play a critical role in killing schistosomula. The short peptides selected with NRS from a 12-mer random phage library have both antigenicity and immunogenicity and two peptides obtained have an identical core sequence. The selected phages stimulate a protective immunity against infection and fecundity of schistosomes. P3 phage clone, as a vaccine candidate, is worthy to be studied further.
Co-first-authors: Min Wang and Xin-Yuan Yi
Co-correspondents: Xin-Yuan Yi
| 1. | Bergquist R, Al-Sherbiny M, Barakat R, Olds R. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002;82:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Capron A, Capron M, Dombrowicz D, Riveau G. Vaccine strategies against schistosomiasis: from concepts to clinical trials. Int Arch Allergy Immunol. 2001;124:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | McManus D. The Schistosoma japonicum angle on vaccine research. Parasitol Today. 2000;16:357-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Yan YT, Liu SX. Advances in the candidate vaccine antigens against Schistosoma japonicum. Zhongguo JiShengChongXue Yu JiShengChongBing ZaZhi. 2000;18:115-119. [PubMed] |
| 5. | McManus DP. A vaccine against Asian schistosomiasis: the story unfolds. Int J Parasitol. 2000;30:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yu XC, Wu GL, Wu YQ, Zhang YJ, Zhang ZH. Studies on the dynamic changes in specific antibodies isotope of IgG IgG2a IgG2c in sera from rats infected with Schistosoma japonicum. Zhongguo Xuexichongbing Fangzhi Zazhi. 2000;12:148-150. |
| 7. | Zhou DM, Yi XY, Zeng XF, Wang M, Zhang SK. Preliminary study on the immunological characteristics of natural resistance in Rat to infection with Schistosoma japonicum. Difangbing Tongbao. 2001;16:25-28. |
| 8. | Vadas MA, Butterworth AE, Sherry B, Dessein A, Hogan M, Bout D, David JR. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. I. Stable and irreversible antibody-dependent adherence. J Immunol. 1980;124:1441-1448. [PubMed] |
| 9. | Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214-218. [PubMed] |
| 10. | van Lieshout L, Stelma FF, Guissé F, Falcao Ferreira ST, Polman K, van Dam GJ, Diakhate M, Sow S, Deelder A, Gryseels B. The contribution of host-related factors to low cure rates of praziquantel for the treatment of Schistosoma mansoni in Senegal. Am J Trop Med Hyg. 1999;61:760-765. [PubMed] |
| 11. | Gryseels B, Mbaye A, De Vlas SJ, Stelma FF, Guissé F, Van Lieshout L, Faye D, Diop M, Ly A, Tchuem-Tchuenté LA. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Trop Med Int Health. 2001;6:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Cêtre C, Pierrot C, Cocude C, Lafitte S, Capron A, Capron M, Khalife J. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713-2719. [PubMed] |
| 13. | Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996;26:1217-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Cêtre C, Cocude C, Pierrot C, Godin C, Capron A, Capron M, Khalife J. In vivo expression of cytokine mRNA in rats infected with Schistosoma mansoni. Parasite Immunol. 1998;20:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Zhou DM, Zeng XF, Wang M, McReynold L, Yi XY. Schistosoma japonicum: preparation of peptides mimicking antigenic epitopes of attenuated cercariae using phage displayed random peptide library. Zhongguo Renshou Gonghuanbing Zazhi. 2002;18:73-75. |
| 16. | Moloney NA, Webbe G. Antibody is responsible for the passive transfer of immunity to mice from rabbits, rats or mice vaccinated with attenuated Schistosoma japonicum cercariae. Parasitology. 1990;100 Pt 2:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Khalife J, Cêtre C, Pierrot C, Capron M. Mechanisms of resistance to S. mansoni infection: the rat model. Parasitol Int. 2000;49:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Chappel JA, He M, Kang AS. Modulation of antibody display on M13 filamentous phage. J Immunol Methods. 1998;221:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Fehrsen J, du Plessis DH. Cross-reactive epitope mimics in a fragmented-genome phage display library derived from the rickettsia, Cowdria ruminantium. Immunotechnology. 1999;4:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Pedersen MV, Køhler LB, Ditlevsen DK, Li S, Berezin V, Bock E. Neuritogenic and survival-promoting effects of the P2 peptide derived from a homophilic binding site in the neural cell adhesion molecule. J Neurosci Res. 2004;75:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Jeon SH, Ben-Yedidia T, Arnon R. Intranasal immunization with synthetic recombinant vaccine containing multiple epitopes of influenza virus. Vaccine. 2002;20:2772-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Stanojević S, Dimitrijević M, Kovacević-Jovanović V, Miletić T, Vujić V, Radulović J. Stress applied during primary immunization affects the secondary humoral immune response in the rat: involvement of opioid peptides. Stress. 2003;6:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Lesinski GB, Smithson SL, Srivastava N, Chen D, Widera G, Westerink MA. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in Balb/c mice. Vaccine. 2001;19:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Misumi S, Endo M, Mukai R, Tachibana K, Umeda M, Honda T, Takamune N, Shoji S. A novel cyclic peptide immunization strategy for preventing HIV-1/AIDS infection and progression. J Biol Chem. 2003;278:32335-32343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Zhou DM, Yi XY, Zeng XF, Wang M, McReynolds L. Immunity against Schistosoma japonicum induced by phage display peptides mimicking antigenic epitopes of Trichinella spiralis. Zhongguo JiShengChongXue Yu JiShengChongBing ZaZhi. 2001;19:268-271. [PubMed] |
| 26. | Jolivet-Reynaud C, Adida A, Michel S, Deléage G, Paranhos-Baccala G, Gonin V, Battail-Poirot N, Lacoux X, Rolland D. Characterization of mimotopes mimicking an immunodominant conformational epitope on the hepatitis C virus NS3 helicase. J Med Virol. 2004;72:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Grabowska AM, Jennings R, Laing P, Darsley M, Jameson CL, Swift L, Irving WL. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology. 2000;269:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Arnon R, Tarrab-Hazdai R, Steward M. A mimotope peptide-based vaccine against Schistosoma mansoni: synthesis and characterization. Immunology. 2000;101:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Fu XM, Zhang ZS, Li CL, Wu HW, Su C, Ji MJ, Wang Y, Liu F, Wu GL. Studies on immunoprotection in mice after immunization with the epitopes of 22.6 kDa antigen of Schistosoma japonicum. Zhongguo Xuexichongbing Fangzhi Zazhi. 2001;13:86-89. |
| 30. | Ouyang L, Yi X, Zeng X, Zhou J, Wang Q, McReynolds L. Partial protection induced by phage library-selected peptides mimicking epitopes of Schistosoma japonicum. Chin Med J (Engl). 2003;116:138-141. [PubMed] |
| 31. | Wang M, Yi XY, Zeng XF, Zhou DM, Yuan SS, Zhang SK, Zhang J. A short peptide mimicking epitopes of the membrane antigen of Shistosoma japonicum hepato-portal schistosomula using phage display techniques. Zhonghua Weishengwuxue He Mianyixue Zazhi. 2003;23:862-865. |