Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2502
Revised: March 4, 2004
Accepted: April 13, 2004
Published online: April 28, 2005
AIM: To develop a cancer vaccine of dendritic cells derived from human cord blood CD34+ cells and to investigate its cytotoxicity on human hepatocarcinoma cells in vitro and in sever combined immunodeficiency (SCID) mice.
METHODS: Lymphocytes from cord blood or peripheral blood were primed by DCs, which were derived from cord blood and pulsed with whole tumor cell lysates. Nonradiative neutral red uptake assay was adopted to detect the cytotoxicity of primed lymphocytes on human hepatocarcinoma cell line BEL-7402 in vitro. The anti-tumor effect of primed lymphocytes in vivo was detected in SCID mice, including therapeutic effect and vaccination effect.
RESULTS: The cytotoxicity of DC vaccine primed lymphocytes from cord blood or peripheral blood on human hepatoc-arcinoma cell line BEL-7402 was significantly higher than that of unprimed lymphocytes in vitro (44.09% vs 14.69%, 47.92% vs 19.44%, P<0.01). There was no significant difference between the cytotoxicity of primed lymphocytes from cord blood and peripheral blood (P>0.05). The tumor growth rate and tumor size were smaller in SCID mice treated or vaccinated with primed lymphocytes than those with unprimed lymphocytes. SCID mice vaccinated with primed lymphocytes had a lower tumor incidence (80% vs 100%, P<0.05) and delayed tumor latent period compared with mice vaccinated with unprimed lymphocytes (11 d vs 7 d, P<0.01).
CONCLUSION: Vaccine of cord blood derived-DCs has an inhibitory activity on growth of human hepatocarcinoma cells in vitro and in SCID mice. The results also implicate the potential role of cord blood derived-DC vaccine in clinical tumor immunotherapy.
-
Citation: Su ZJ, Chen HB, Zhang JK, Xu L. Effects of dendritic cells from cord blood CD34+ cells on human hepatocarcinoma cell line BEL-7402
in vitro and in SCID mice. World J Gastroenterol 2005; 11(16): 2502-2507 - URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2502.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2502
Hepatocarcinoma is a major malignant cancer in Asian and southern African countries. Resection, chemotherapy and alcohol injection are potentially curative for only small, local tumors. Unfortunately, the diseases of most patients have developed to the advanced stage at diagnosis. In China, the mortality of hepatocarcinoma ranks second among malignant tumors, only behind that of lung cancer in urban areas and stomach cancer in rural areas[1].
Dendritic cells (DCs) express abundant MHC molecules and costimulatory molecules, and are professional antigen presenting cells (APC). Immature DCs can uptake antigens through macropinocytosis, phagocytosis and receptor-mediated endocytosis and have a poor ability to activate lymphocytes. After capturing antigens, immature DCs come into mature stage and migrate from peripheral areas to lymph nodes, where they present antigens to lymphocytes and activate the immune response. For their potent ability to present antigens and activate lymphocytes, DCs are considered as the natural adjuvant for immune response[2,3]. Not surprisingly, tumor cells could escape from host immune surveillance when there is a functional failure of DCs, and the fact is that the number of mature DCs with normal functions was decreased in local tumor tissue and peripheral blood of tumor patients[4,5]. Based on these, DC-based experiments have been developed for tumor immunotherapy in the past decade. Our laboratory once found that DCs from mice spleen fused with hepatocarcinoma cells that manifested antitumor effects in vivo. There are also reports on DCs from human peripheral blood generating specific T cell responses and eliciting tumor regression[6,7]. However, getting a great deal of peripheral blood or other tissues from tumor patients to produce DCs in vitro is not practical in clinics. Since peripheral blood DCs are derived from bone marrow CD34+ multipotential hematopoietic stem cells in vivo, Ferrari et al[8], adopted chemotherapy combined with granulocyte colony-stimulating factors to mobilize bone marrow CD34+ cells into peripheral blood of cancer patients, and they found with such results that there was an increase of CD34+ cells in peripheral blood, but the number of DCs was not significantly increased, and the subset of DCs changed, i.e., the ratio of DC1/DC2 reversed, implicating that the differentiation of DCs in cancer patients was dysfunctional.
In cord blood, the percentage of CD34+ cells is slightly lower than in bone marrow, but the percentage of CD34+/CD38- cells is higher. Compared with CD34+/CD38+ cells, CD34+/CD38- cells are early stem cells and more sensitive to stimulation of cytokines[9,10]. In addition, harvesting cord blood is a slightly invasive procedure. Here, we investigated the anti-tumor effects of DCs from human cord blood CD34+ cells on human hepatocarcinoma cell line BEL-7402 in vitro and in sever combined immunodeficiency (SCID) mice.
Human hepatocellular carcinoma cell line BEL-7402 (Shanghai Institute of Cell Biology, Chinese Academy of Sciences) was cultured in RPMI 1640 (GIBCO BRL, USA) containing 10% heat-inactivated newborn calf serum (NCS, GIBCO BRL, USA), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere containing 50 mL/L CO2. SCID mice (6-8-wk old) were purchased from Animal Center of Sun Yat-Sen University Medical College (Guangzhou, China), bred in a specific-pathogen-free laboratory at Shantou University Medical College Affiliated Tumor Hospital. All animal procedures were performed in accordance with recommendations for the proper use and care of laboratory animals.
Normal human umbilical cord blood was obtained from Department of Obstetrics of the First Affiliated Hospital of Shantou University Medical College. Fresh cord blood samples were diluted 1:4 in PBS (pH 7.4). After Ficoll-Hypaque (1.077 g/mL, Tianjin Hematology Institute, China) centrifugation (400 r/min for 35 min at 20 °C), mononuclear cells were collected from the interface and washed twice in PBS (200 r/min for 10 min at 20 °C).
Cord blood CD34+ hematopoietic stem cells were labeled with a CD34+ progenitor cell isolation kit (Miltenyi Biotec, Germany) and separated from cord blood mononuclear cells by Mini magnetic cell sorting (Mini MACS, Miltenyi Biotec, Germany) according to the protocol. DCs were induced from cord blood CD34+ hematopoietic stem cells as previously described[11]. Briefly, CD34+ cells were adjusted to the concentration of 3×104/mL and cultured at 37 °C in a humidified 50 mL/L CO2 atmosphere in RPMI 1640 containing 10% NCS, 100 U/mL penicillin, 100 μg/mL streptomycin and supplemented with 100 μg/mL recombined human granulocyte-macrophage colony-stimulating factor (rhGM-CSF, Biotinge Co., China), 50 U/mL recombined human tumor necrosis factor-α (TNF-α, PeproTech EC Ltd Co., UK). For every 2 d, half of the medium was replaced with fresh complete medium supplemented with 100 μg/mL rhGM-CSF, 50 U/mL TNF-α for a total period of 2 wk. The differentiation process of DCs was observed under a phase contrast microscope (Olympus, Japan).
Human hepatocarcinoma cell line BEL-7402 was trypsinized and washed twice in PBS, then lysed by ultrasonication (Misonix, USA). The cell lysates were monitored by light microscopy. After being cultured with GM-CSF and TNF-α for 14 d, cord blood-derived DCs were collected and incubated with whole cell lysates of BEL-7402 at a ratio of three tumor cells equivalent to one DC (i.e., 3:1) in RPMI 1640 containing 10% NCS. After 12 h of incubation, pulsed DCs were washed twice in PBS and resuspended in RPMI 1640.
Human peripheral blood (from healthy volunteers of our laboratory) or cord blood (from Department of Obstetrics of the First Affiliated Hospital of Shantou University Medical College) mononuclear cells were isolated with Ficoll-Hypaque by density gradient centrifugation and cultured in RPMI 1640 complete medium. After being incubated for 4 h, non-adherent cells were harvested as lymphocytes.
Cord blood derived-whole tumor cell lysate pulsed-DCs were added to lymphocytes from peripheral blood or cord blood at a responder-to-stimulator ratio of 20:1 in RPMI 1640 containing 10% NCS and 80 U/mL recombined human interleukin-2 (rhIL-2, Biotinge Co., China). After being cultured for 5 d, cells were harvested as primed lymphocytes.
Human hepatocarcinoma cell line BEL-7402 was seeded into 96-well cell culture plates (Costar, USA) at a concentration of 0.4×104/200 μL per well. After 24-h incubation, the culture medium was removed and the cell number was counted. Then fresh medium containing DC-primed lymphocytes from peripheral blood, DC-primed lymphocytes from cord blood, unprimed lymphocytes from peripheral blood, unprimed lymphocytes from cord blood were added as effector cells in PB-DLC group, CB-DLC group, PB-LC group and CB-LC group respectively, at an effector-to-target ratio of 10:1, three wells for every group. In control group, no effector cells were added. Forty-eight hours after the effector cells were added, the medium was replaced by 100 μL neutral red (30 μg/mL). After incubation for another 1 h, the neutral red was removed. Cultures were carefully washed twice with PBS and the neutral red in tumor cells was extracted with 100 μL solution of 1% hydrochloric acid/ethanol. Neutral red absorbance value (A value) of tumor cells at wavelength 570 nm was detected with a 3550-UV microplate reader (Bio-Rad, USA). Each assay was performed in quadruplicate. The results were presented as mean±SD. Cytolytic activities of the effector cells were calculated as (1-A value of experimental group/A value of control group)×100%.
Tumor cells and SCID mice were prepared as described above. To study the therapeutic effect of DC-primed lymphocytes on hepatoma in vivo, a human hepatoma model was established in SCID mice by subcutaneous (s.c.) injection of viable BEL-7402 of logarithmic stage (2×106 cells in 100 μL PBS for each mouse) at the right flank. Subsequently, the tumor-bearing mice in Tr-DLC group and Tr-LC group (5 mice/group) were injected s.c. at the left flank with 1×107 DC-primed lymphocytes or with unprimed lymphocytes from peripheral blood respectively, twice at 3-d intervals.
To study the protective effect of DC-primed lymphocytes on human hepatocarcinoma cells in vivo, SCID mice in Im-DLC group and Im-LC group (5 mice/group) were first vaccinated with 1×107 DC-primed lymphocytes or with unprimed lymphocytes respectively, twice at 3-d intervals. Four days after the second vaccination, the mice in Im-DLC group and Im-LC group were both challenged with human hepatocarcinoma BEL-7402 cells of logarithmic stage (2×106 cells in 100 μL PBS for each mouse).
In control group, SCID mice were just injected s.c. with human hepatocarcinoma BEL-7402 cells (2×106) at the right flank. After tumor cell injection, tumor incidence and tumor latent period of all mice were recorded. The widest diameter (a) and narrowest diameter (b) of the tumor in vivo were measured twice a week with calipers. Tumor volume was calculated according to the formula V = ab2/2[12]. After 32 d of tumor cell injection, all the mice were killed and tumor volume was measured ex vivo.
Data were analyzed with SPSS11.0 statistical software. Tumor volumes were statistically analyzed after evolution. The significant difference between groups was determined by one-way ANOVA test. Two-sided P<0.05 was considered statistically significant. No mouse was excluded from this study.
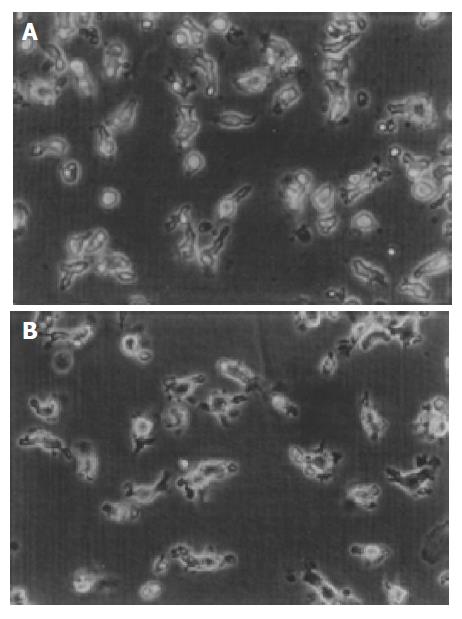
CD34+ cells from cord blood were round and regular with a diameter of about 7-8 μm. Under the stimulation of rhGM-CSF 100 μg/mL and rhTNF-α 50 U/mL, the cell number increased and cell clones formed. At the same time the cells stretched out cytoplasmic projections. During the later period of culture, DCs shed from the clones to the medium. On day 14, the total number of cells increased about 20-fold. Under a phase contrast microscope, we found that after being pulsed with BEL-7402 cell lysates at a ratio of 3:1, human cord blood-derived DCs displayed more pronounced dendritic processes (Figure 1).
Lymphocytes were primed by tumor cell lysate pulsed-DCs at a responder-to-stimulator ratio of 20:1 for 5 d. As shown in Table 1, the neutral red absorbance value of viable tumor cells in PB-DLC group and CB-DLC group was significantly lower than that in PB-LC and CB-LC groups (P<0.01). There was no significant difference in A value between PB-DLC group and CB-DLC group (P>0.05). According to A value, the cytotoxicity of primed lymphocytes from peripheral blood or cord blood was 47.92% and 44.09% respectively, while the cytotoxicity of unprimed lymphocytes was 19.44% and 14.69%.
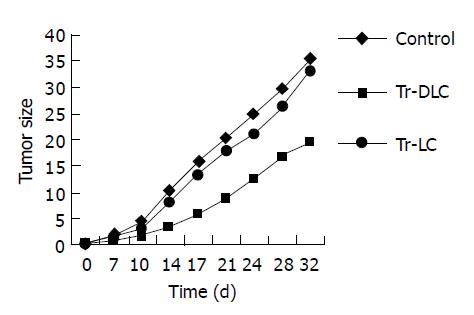
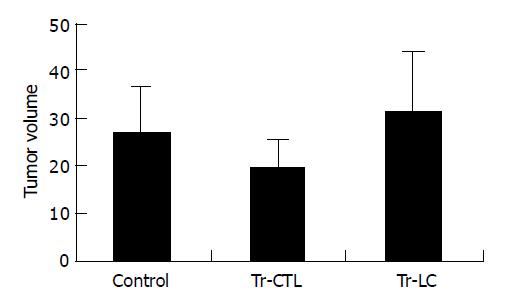
To evaluate whether DCs derived from cord blood had therapeutic effects on hepatoma cells in vivo, treatment experiments were performed in SCID mice. All SCID mice developed tumors within 7 d after injection of 2×106 hepatocarcinoma BEL-7402 cells. In tumor-bearing mice treated with DC-primed lymphocytes, both the tumor growth rate in vivo (Figure 2) and the tumor volume measured ex vivo on the 32nd d after tumor cell injection (Figure 3) were smaller than those in mice treated with unprimed lymphocytes (P<0.05). There was no significant difference between mice treated with unprimed lymphocytes and mice in control group (P>0.05) (Figures 2 and Figures 3).
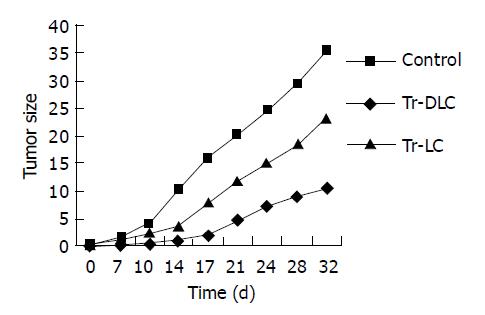
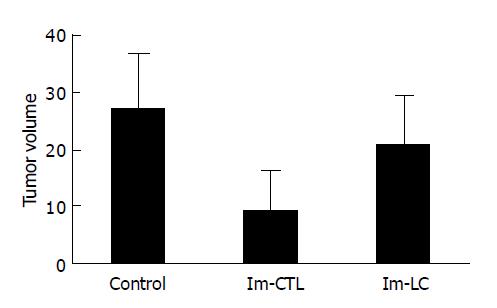
To evaluate whether DCs derived from cord blood exerted protective effects in SCID mice against human hepatoma cells in vivo, vaccination experiments were performed. All mice (5/5) vaccinated with unprimed lymphocytes developed tumors within 7 d after injection of 2×106 hepatocarcinoma cells. On day 32 after tumor challenge, 80% (4/5) mice vaccinated with DC-primed lymphocytes developed tumors and the tumor latent period was about 11-14 d. Tumor growth rate in vivo (Figure 4) and tumor volume measured ex vivo on the 32nd d after tumor cell challenge (Figure 5) both decreased in mice vaccinated with DC-primed lymphocytes compared with mice vaccinated with unprimed lymphocytes and mice in control group (P<0.05).
In this study, CD34+ cells were isolated from cord blood mononuclear cells by Mini MACS. With the stimulation of rhGM-CSF and rhTNF-α, cord blood-derived CD34+ cells could proliferate, and the cell number and formation of cell clones were increased. In this experiment, the number of total cells increased about 20-fold after being cultured for 14 d with cytokines. During proliferation, cells differentiated into DCs and displayed cell projections. Caux et al[11], reported that cord blood CD34+ cells differentiated into DCs in two different ways with the stimulation of GM-CSF and TNF-α. After being cocultured with cytokines for 5-7 d, there were two kinds of cell phenotypes, one was CD14+, the other was CD1a+. On the 12-14th d of culture, they both differentiated into DCs.
After being pulsed with whole tumor cell lysates, cord blood-derived DCs manifested more processes, which were similar to the morphological changes of Langerhans cells from skin stretching out typical dendrites after being cultured for several days in vitro. Researchers also found that formation of cell dendrites was related to the expression of cell skeleton 55 ku fascin-actin bundling protein[13]. Fascin and CD83 were also the phenotypic characteristics of mature DCs[14,15]. We considered that the typical dendrites might be the morphological characteristics of mature DCs.
Since neutral red can stain viable cells and is rejected by dead cells, we adopted the nonradiative neutral red uptake assay in this study to detect the absorbance value of tumor cells, and then the cytolytic activity of effector cells on tumor cells was calculated according to the absorbance value. The results in Table 1 indicated that the cytotoxicity of primed lymphocytes was significantly higher than that of unprimed lymphocytes from peripheral blood in vitro, so were primed lymphocytes and unprimed lymphocytes from cord blood. Cord blood lymphocytes mainly consist of CD45RA+ naive cells[16]. Our finding in this study that there was no significant difference between the cytolytic activity of primed lymphocytes from cord and peripheral blood suggested that DCs from cord blood respectively could activate naive lymphocytes as well as memory lymphocytes, which was consistent with previous reports[17].
The potent effect of DCs on activating resting lymphocytes was associated with its surface molecules and cell skeleton. One of these molecules attracting the attention of many researchers is DC-SIGN (DC-specific ICAM-3 grabbing nonintegrin), which is the receptor of HIV and mycobacteria, more importantly, also the high affinitive receptor of intercellular adhesion molecule (ICAM)-3 expressed on lymphocytes[18-20]. DC-SIGN can promote DCs to contact with lymphocytes through combination with ICAM-3, even without the existence of antigens. Moreover, the contact of DCs with lymphocytes and formation of immunosynapse are necessary for DCs presenting antigens and activating lymphocytes. Cell skeleton protein fascin not only takes part in maintaining cell morphology and developing cell dendrites but also plays an important role in the active skeleton rearrangement and formation of immunosynapse[14,21]. Mosialos et al[22], and Kupfer and Singer[23] reported that DCs were the only leukocytes that could express fascin and the only APC that could make an active rearrangement of its skeleton during the formation of immunosynapse. It is possible that the DC-specific DC-SIGN and the ability of skeleton rearrangement are associated with its specific ability to activate resting lymphocytes.
In this study, we adopted SCID mice, which lacked T and B lymphocytes genetically, to study the antitumor effect of DC primed lymphocytes in vivo. The sections stained with HE showed that spleen cells of SCID mice were scattered with no obvious splenic corpuscle or periarterial lymphatic sheath (picture not shown). This indicated that SCID mice in this experiment had no “immune leak”, so there was no host immune response to injected cells. In SCID mice treated with DC-primed lymphocytes, the rate of tumor growth was slower than that of mice in control group, suggesting that DC vaccine can inhibit tumor growth in vivo. While in mice vaccinated with DC-primed lymphocytes, and challenged with human hepatocarcinoma cells, though still some mice developed tumors, tumor incidence was significantly decreased, implicating the antitumor effect of DC vaccine in vivo. The reason why the tumor volume in mice treated with unprimed lymphocytes was greater than that in control group may be the heterogeneity of individuals. After all, the difference between these two groups was not significant (P>0.05).
The rejection of tumor cells in vivo depends on effector T cells. In the results of our experiments, tumor cell lysates pulsed-DC vaccine manifested some therapeutic and protective effects against tumor cells in vivo. The use of tumor cell lysates as a source of antigens to pulse DCs has several advantages. First, it mimics the physiologic process by which a growing tumor induces an immune response. Second, there is also a possibility that DCs pulsed with whole tumor cell lysates could present a broader range of epitopes of tumor antigens and activate polyclone subpopulations of T cells compared to a single peptide or antigen-pulsed DCs. Third, it is not necessary to characterize and isolate tumor molecular antigens. The preparation of such a DC vaccine pulsed with whole tumor cell lysates is relative facile. One disadvantage of this approach, however, is the potential danger of evoking autoimmune reactivity to self or normal tissues. Anti-dsDNA and anti-nuclear antibodies were found existing in animal experiments after vaccinated with whole tumor antigen-pulsed DCs[24,25]. In clinic, fortunately, no unbearable side effects have been reported except for rare vitiligo in melanoma patients treated with whole tumor antigen-pulsed DCs[26]. Many clinic experiments have also proved that DC vaccine is safe for patients. We wish this preliminary study can provide some evidence for advanced study of cord blood-derived DC vaccine and its application in clinics for tumor patients.
We thank Miss Jiong-Yu Chen and Professor De-Rui Li of Affiliated Tumor Hospital, Professor Kang-Sheng Li of Department of Immunology and staff of Department of Obstetrics and Gynecology in First Affiliated Hospital of Shantou University Medical College, for their assistance and support to this work.
| 1. | Zhang S, Li L, Lu F. Mortality of primary liver cancer in China from 1990 through 1992. Zhonghua ZhongLiu ZaZhi. 1999;21:245-249. [PubMed] |
| 2. | Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1675] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 4. | Schwaab T, Weiss JE, Schned AR, Barth RJ. Dendritic cell infiltration in colon cancer. J Immunother. 2001;24:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mulé JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513-8519. [PubMed] |
| 7. | Geiger J, Hutchinson R, Hohenkirk L, McKenna E, Chang A, Mulé J. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet. 2000;356:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ferrari S, Rovati B, Porta C, Alessandrino PE, Bertolini A, Collovà E, Riccardi A, Danova M. Lack of dendritic cell mobilization into the peripheral blood of cancer patients following standard- or high-dose chemotherapy plus granulocyte-colony stimulating factor. Cancer Immunol Immunother. 2003;52:359-366. [PubMed] |
| 9. | Ueda T, Yoshida M, Yoshino H, Kobayashi K, Kawahata M, Ebihara Y, Ito M, Asano S, Nakahata T, Tsuji K. Hematopoietic capability of CD34+ cord blood cells: a comparison with CD34+ adult bone marrow cells. Int J Hematol. 2001;73:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Gigant C, Latger-Cannard V, Bensoussan D, Feugier P, Bordigoni P, Stoltz JF. Quantitative expression of adhesion molecules on granulocyte colony-stimulating factor-mobilized peripheral blood, bone marrow, and cord blood CD34+ cells. J Hematother Stem Cell Res. 2001;10:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 693] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 12. | Carlsson G, Gullberg B, Hafström L. Estimation of liver tumor volume using different formulas - an experimental study in rats. J Cancer Res Clin Oncol. 1983;105:20-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Ross R, Ross XL, Schwing J, Längin T, Reske-Kunz AB. The actin-bundling protein fascin is involved in the formation of dendritic processes in maturing epidermal Langerhans cells. J Immunol. 1998;160:3776-3782. [PubMed] |
| 14. | Al-Alwan MM, Rowden G, Lee TD, West KA. Fascin is involved in the antigen presentation activity of mature dendritic cells. J Immunol. 2001;166:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Thurnher M, Papesh C, Ramoner R, Gastl G, Böck G, Radmayr C, Klocker H, Bartsch G. In vitro generation of CD83+ human blood dendritic cells for active tumor immunotherapy. Exp Hematol. 1997;25:232-237. [PubMed] |
| 16. | D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197-203. [PubMed] |
| 17. | Caux C, Massacrier C, Dezutter-Dambuyant C, Vanbervliet B, Jacquet C, Schmitt D, Banchereau J. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+ T cells and process soluble antigen. J Immunol. 1995;155:5427-5435. [PubMed] |
| 18. | Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1854] [Cited by in RCA: 1840] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 19. | Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1300] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 20. | Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 494] [Cited by in RCA: 468] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus GS, Kieff E, Langhoff E. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am J Pathol. 1996;148:593-600. [PubMed] |
| 23. | Kupfer A, Singer SJ. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989;170:1697-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ludewig B, Ochsenbein AF, Odermatt B, Paulin D, Hengartner H, Zinkernagel RM. Immunotherapy with dendritic cells directed against tumor antigens shared with normal host cells results in severe autoimmune disease. J Exp Med. 2000;191:795-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Bondanza A, Zimmermann VS, Dell'Antonio G, Dal Cin E, Capobianco A, Sabbadini MG, Manfredi AA, Rovere-Querini P. Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol. 2003;170:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 217] [Article Influence: 7.8] [Reference Citation Analysis (0)] |