Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1833
Revised: March 10, 2004
Accepted: April 16, 2004
Published online: March 28, 2005
AIM: To develop an oral attenuated Salmonella typhimurium vaccine against gastric cancer and to evaluate its efficacy in mice.
METHODS: A complementary sequence of Nco I site and a sequence coding for MG7-Ag mimotope were designed at the 5’ terminus of forward primer. Using p1.2 II-HBCAg plasmid as template, PCR was performed to get a fusion gene of the mimotope and a HBcAg gene. The fusion gene was then subcloned into the plasmid pYA3341 complementary to Salmonella typhimurium X4550, and the recombinant plasmid was then transformed into attenuated Salmonella typhimurium X4550. Balb/c mice were orally immunized with the recombinant Salmonella typhimurium X4550. The mice were immunized every 2 wk to reinforce the immunity. At the 6th wk, serum titer of antibody was detected by ELISA, and at the 8th wk, cellular immunity was detected by 51Cr release test. Ehrlich ascites carcinoma cells expressing MG7-Ag were used in tumor challenge assay as a model to evaluate the protective effect of the vaccine.
RESULTS: Serum titer of antibody against MG7-Ag was significantly higher in mice immunized with the vaccine than in control groups (0.9538±0.043 vs 0.6531±0.018, P<0.01; 0.9538±0.043 vs 0.6915±0.012, P<0.01), while in vitro51Cr release assay of the splenocytes showed no statistical difference in the three groups. Two weeks after tumor challenge, 1 in 5 immunized mice was tumor free, while all the mice in the control group presented tumor.
CONCLUSION: Oral attenuated Salmonella typhimurium vaccine against the MG7-Ag mimotope of gastric cancer is immunogenic. It can induce significant humoral immunity against tumors in mice, and has some protective effects.
-
Citation: Meng FP, Ding J, Yu ZC, Han QL, Guo CC, Liu N, Fan DM. Oral attenuated
Salmonella typhimurium vaccine against MG7-Ag mimotope of gastric cancer. World J Gastroenterol 2005; 11(12): 1833-1836 - URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1833.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1833
Gastric cancer is the most common malignant tumor in China and the second most common malignancy in the world. Conventional intervention measures such as operation and chemotherapy work poorly in treating gastric cancer. With few tumor-specific antigens identified, there have been no effective vaccines developed to combat gastric cancer. MG7-Ag, discovered by our institute, is a kind of gastric cancer-specific tumor associated antigens specifically expressed in gastric carcinomas and can be used as an indicator of high risk of malignant changes in gastric mucosa dysplasia[1]. Primary studies showed that MG7-Ag could elicit significant and specific immune response against gastric cancer, suggesting that it could be an excellent target for cancer vaccine development[2]. However, due to its unknown identity, it is rather difficult to isolate and purify the MG7-Ag from tumor tissues. Recently, we have identified the mimotopes of MG7-Ag by screening the phage display library, and the mimotopes could mimic the primary antigen efficiently, as shown by in vitro and in vivo assays[3]. The present study was to develop an oral attenuated Salmonella typhimurium vaccine using the MG7-Ag mimotope of gastric cancer.
Attenuated Salmonella typhimurium X4550(ρcrp-1, ρcya-1, asd-, NA+, R-M+) , E.coli X6212 (asd-, NA+, R-M+) and plasmid pYA3341(asd+) were a gift from Dr. Curtiss (Washington University, St.Louis, USA). Plasmid p1.2 II and E.coli DH5 α were from Institute of Digestive Disease, Xijing Hospital, Fourth Military Medical University, Xi’an, China.
A pair of PCR primers (P1.1, P1.2) was designed by using Primer Premier 5.0 software. Sense primers (P1.1): 5’-TGCCATGGGAAAACCGCACGTTCACACTAAAGGTGGTGG-TTCTCTTGGGTGGCTTTGGGGC-3’, contained Nco I digestion site, ATG and MG7-Ag mimotope. Reverse primer P1.2: 5’- CCAAGCTTCTAACATTGAGATTCCCG-3’, contained Hind III digestion site. Plasmid p1.2 II was used as template. The PCR product was visualized in agarose electrophoresis and then cloned into pUCm-T vector and sequenced on ABI PRISMTM 377 sequencer. Then, the PCR product was subcloned into pYA3341 (asd+) vector from the pUCm-T vector by restrictive enzyme digestion with Nco I and Hind III, the recombinant plasmid was named pYA3341-MG7/HBcAg. The vector was sequenced to confirm the proper coding sequence.
Recombinant plasmid pYA3341-MG7/HBcAg was transformed into E.coli X6212 (asd-, NA+, R-M+) by CaCl2 protocol. After being modified in E.coli X6212 (asd-, NA+, R-M+), pYA3341-MG7/HBcAg was extracted and transformed into S. typhimurium X4550 (asd-, NA+, R+M+) by electroporation (2.5 kV, 25 μF, 200 Ω, pulse time 0.0326 s). Recombinant plasmid pYA3341-MG7/HBcAg was extracted from S. typhimurium X4550 and digested with Nco I and Hind III to confirm the sequence.
Recombinant Salmonella typhimurium X4550 cells were grown with aeration for 12 h in LB and lysed in 1 mg/L sodium dodecyl sulfate (SDS) for 5 min at 65 °C.Protein content of each sample was estimated by bicinchoninic acid protein assay, and adjusted to 500 μg of protein per mL with water followed by dilution in 2×loading buffer, and the sample was placed in boiling water for 3 min. Protein samples (40 μg per lane) were separated by 12% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes with a semidry transfer apparatus, and blocked in 5% BSA in PBS. Anti-MG7 antibody used as primary antibody was detected with horseradish peroxidase-conjugated goat anti-mouse secondary antibody and detected using DAB.
Thirty 4-wk-old female Balb/c mice weighing 15-20 g were used in the immunization assay. They were randomly divided into 3 groups, which were respectively given oral PBS solution (10 mice, PBS control), attenuated Salmonella typhimurium X4550 containing empty pYA3341 plasmid (10 mice in empty group) or the empty pYA3341-MG7/HBcAg (10 mice in immunization group). Before immunization, all the mice were fasted overnight and pre-administered with 100 μL 10 g/L NaHCO3 solution. Each time, 100 μL pH7.6 PBS was given to the mice in PBS control group, and 1×108Salmonella typhimurium X4550 were given to the mice in the empty control and immunization groups. PBS and Salmonella typhimurium X4550 were given to the mice by orogastric inoculation. Immunization was repeated every two weeks. At the 6th wk after the first immunization, sera from the mice were prepared and 1:80 diluted. By coating KATO III cells expressing MG7-Ag on the plates, cellular ELISA was performed to detect the antibody against MG7-Ag. At the 8th wk, the splenocyte suspension was prepared, and 51Cr release assay[4] was performed to test the cellular immunity. Briefly, 1×106 Ehrlich ascites carcinoma cells (EAC) were incubated with Na51CrO4 (70 μCi) in an incubator at 37 °C, 50 mL/L CO2 for 4 h. Then, 1×104 cells (100 μL) were plated into each well of a 96-well plate, and 1×104 splenocytes were added. Both cells were incubated in an incubator at 37 °C, 50 mL/L CO2 for 4 h. The plates were harvested, and the killing response of splenocytes was examined by measuring the β counts of the cells.
To further investigate the efficacy of the oral S. typhimurium X4550 vaccine, tumor challenge assay was performed. Briefly, 1×108 Ehrlich ascites carcinoma cells (EAC) were injected into the abdominal cavity of the mice (5 mice in each group). Tumor masses were isolated and weighed and the survival rate of each group was observed.
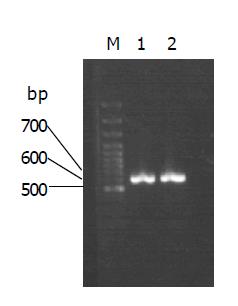
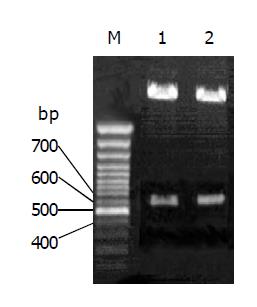
By PCR, MG7-Ag mimotope and HBcAg were fused together and incorporated into a fragment of the pUCm-T (Figure 1). The proper coding of the epitopes was confirmed by sequencing. The PCR product was subcloned into pYA3341. Th Hind III and Nco I digestion, the fragment of the fused gene were confirmed (Figure 2).
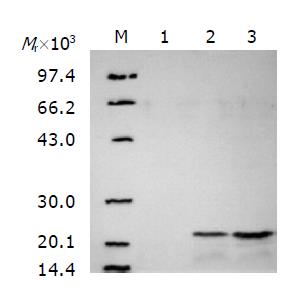
Western blot showed that the expression product of recombinant plasmid pYA3341-MG7/HBcAg in the X4550 displayed a protein band, with Mr about 22000, bound specifically to anti-MG7mAb (Figure 3).
Immediately after orogastric inoculation of the vaccine, mice presented rapid shallow breathing, slow reaction and decreased activity. After about 5-20 min, the above symptoms improved, and the mice recovered completely after 2-3 h. No diarrhea was seen in the mice given Salmonella typhimurium containing either recombinant pYA3341-MG7/HBcAg or empty vector, and no mouse in all groups died after immunization.
Six weeks after the first immunization, serum titer of the antibody against MG7-Ag was significantly increased in the vaccine-immunized mice, while no significant MG7 antibody increase was detected in 2 control groups. There was a significant difference between the vaccine-immunized group and 2 control groups respectively (P<0.01), while no difference was detected between the two control groups (P>0.05, Table 1). The result of 51Cr release assay showed no statistical difference in the three groups (Table 2), suggesting that no significant cellular immunity was elicited.
All mice challenged with Ehrlich ascites cells were observed for tumor growth. Twenty-five days after the cell challenge, the tumor mass formation was seen in all mice of the three groups. The average tumor weight in vaccinated group was markedly smaller than that in two control groups (P<0.05), there was no significant difference in the tumor weight between the two control groups (P>0.05,Table 3). Tumor challenge assay showed that 1 out of 5 immunized mice had no tumor, and none in the control group was protected.
Gastric cancer is the leading cause of cancer death in China. Biotherapy has become a new way for humans to combat gastric cancer. Carbohydrate antigens are the most abundant antigens expressed on cancer cell surface and seem to play a role in cancer etiology and prognosis. Therefore, carbohydrates are considered as important targets for immunological and pharmacological intervention. However, they are typically poorly immunogenic, difficult to be purified and synthesized in large quantities, and usually result in mostly short-lived IgM-type antibodies in a vaccinated host without long-lasting immunity. Additionally, most carbohydrate antigens are T-cell independent, indicating their inability to stimulate MHC class II-dependent T-helper cells[4]. Consequently, carbohydrates are not capable of inducing a sufficient anamnestic or secondary immune response. In order to overcome these weaknesses, an alternative approach to the development of carbohydrate-based vaccines is to use peptide or polypeptide surrogates that could mimic carbohydrate antigen[5]. Anti-idiotypic antibodies[6,7] and peptides[8-10] that could mimic tumor-associated carbohydrate antigens have been described. MG7-Ag of gastric cancer was discovered by our institute, and its immunogenicity was found to come from the carbohydrate chain of a glycoprotein. Due to its unknown identity, MG7-Ag is hard to be isolated from tumor tissues. In our previous study, we employed phage-displaying technology to obtain a series of oligopeptides that could mimic gastric cancer-associated antigen (MG7-Ag).
In order to investigate the immunogenicity of the mimotope, we constructed a recombinant gene vaccine of MG7-Ag mimotope fused with HBcAg. The particulate HBcAg is extremely immunogenic and can function both as a T-cell-dependent and T-cell-independent antigen. Immunization with HBcAg could preferentially prime the Th1-type cellular immune response[11-14]. During chronic HBV infection, HBcAg is the only antigen known to elicit a prominent immune response[15]. In addition, HBcAg is an effective carrier for heterologous peptide epitopes including the HBV surface antigen preS1 and could enhance the immunogenicity of the antigens that it carries[16,17]. While fusion to the N-terminus requires a linker to become surface accessible, both fusions to the N-terminus and to the C-terminus were compatible with particle assembly and preserve the native antigenicity and immunogenicity of HBcAg. Fusion to an immunodominant internal site of HBcAg reduces the HBcAg immunogenicity and antigenicity and most drastically enhance the immunogenicity of the inserted foreign epitope[18]. In this study, we fused MG7 to the N-terminus of HBcAg with a linker LGWL.
Attenuated strains of Salmonella typhimurium have been widely used as vehicles for delivery and expression of vaccine antigens. Attenuated Salmonella typhimurium strains expressing antigens from bacteria, viruses and parasites have been proved efficient as well as safe in combating respective pathogens[19].The ρcyaρcrp Salmonella vaccine strains have been shown to be both avirulent and immunogenic in mice[20], and introduction of the Asd+ plasmid into the ρasd Salmonella mutants could completely restore avirulence. These mutants express high levels of cloned gene products and are very stable both in vivo and in vitro, without adverse effects on the growth of bacteria. When used as an oral vehicle, they can invade M cells and intestinal epithelial cells and penetrate the mucosal barrier of the intestine. They can be uptaken by macrophages and dendritic cells (DC) in the lamina propria and the Peyer’s patch. Intracellular bacteria would not undergo lysis in the lysosomes immediately but survive for a period of time due to unknown reasons. They could provide a reservoir of antigens, so these features of Salmonella typhimurium strains make them an excellent vehicle of oral vaccines.
In our study, serum titer of antibody against MG7-Ag was significantly higher in mice immunized with the vaccine than in control groups, but there was no significant and specific T cell response. Its absence might be due to the immunogenic type of the primary antigen. However, the oral attenuated Salmonella typhimurium vaccine developed by us was shown to be partially protective as demonstrated by the tumor challenge assay, though no significant difference was seen in the 51Cr release assay. The inconsistency between the results of tumor challenge assay and 51Cr release assay might be due to the following reasons: One is that the 51Cr release assay was inaccurate in determining the T cell response. More accurate methods include ELISPOT and others. Moreover in our study, we did not isolate the CD8+ T cells while performing the 51Cr release assay. The existence of other types of cells might affect the results. The other is that the sample included in the tumor challenge assay was too small. Much larger samples would be needed to further confirm the protective effect of the oral attenuated Salmonella typhimurium vaccine.
In conclusion, the vaccine against MG7-Ag mimotope is immunogenic and can induce a significant immune response against gastric cancer and may be partially protective in mice. Our results have verified the feasibility of developing an oral Salmonella typhimurium vaccine against the MG7-Ag mimotopes, its efficacy as well as safety.
| 1. | Liu J, Hu JL, Zhang XY, Qiao TD, Chen XT, Wu KC, Ding J, Fan DM. The value of MG7 antigen in predicting cancerous change in dysplastic gastric mucosa. Int J Clin Pract. 2002;56:169-172. [PubMed] |
| 2. | Xu L, Qiao T, Chen B. Mimic epitope recognized by monoclonal antibody MG7 against gastric cancer through screening phage displayed random peptide library. Zhonghua YiXue ZaZhi. 2000;80:304-307. [PubMed] |
| 3. | Xu L, Xu H, Ma F. Immunogenicity of phage-displayed tumor antigen-mimic peptide. Zhonghua ZhongLiu ZaZhi. 2001;23:187-189. [PubMed] |
| 4. | Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 605] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Shikhman AR, Cunningham MW. Trick and treat: toward peptide mimic vaccines. Nat Biotechnol. 1997;15:512-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Furuya A, Yoshida H, Hanai N. Development of anti-idiotype monoclonal antibodies for Sialyl Le(a) antigen. Anticancer Res. 1992;12:27-31. [PubMed] |
| 7. | Chapman PB. Anti-idiotypic monoclonal antibody cancer vaccines. Semin Cancer Biol. 1995;6:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Qiu J, Luo P, Wasmund K, Steplewski Z, Kieber-Emmons T. Towards the development of peptide mimotopes of carbohydrate antigens as cancer vaccines. Hybridoma. 1999;18:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kieber-Emmons T, Luo P, Qiu J, Chang TY, O I, Blaszczyk-Thurin M, Steplewski Z. Vaccination with carbohydrate peptide mimotopes promotes anti-tumor responses. Nat Biotechnol. 1999;17:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Charalambous BM, Feavers IM. Mimotope vaccines. J Med Microbiol. 2001;50:937-939. [PubMed] |
| 11. | Bertoletti A, Chisari FV, Penna A, Guilhot S, Galati L, Missale G, Fowler P, Schlicht HJ, Vitiello A, Chesnut RC. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J Virol. 1993;67:2376-2380. [PubMed] |
| 12. | Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442-3449. [PubMed] |
| 13. | Bertoletti A, Southwood S, Chesnut R, Sette A, Falco M, Ferrara GB, Penna A, Boni C, Fiaccadori F, Ferrari C. Molecular features of the hepatitis B virus nucleocapsid T-cell epitope 18-27: interaction with HLA and T-cell receptor. Hepatology. 1997;26:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Milich DR, Schödel F, Hughes JL, Jones JE, Peterson DL. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192-2201. [PubMed] |
| 15. | Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 1997;94:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Schödel F, Moriarty AM, Peterson DL, Zheng JA, Hughes JL, Will H, Leturcq DJ, McGee JS, Milich DR. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J Virol. 1992;66:106-114. [PubMed] |
| 17. | Clarke BE, Brown AL, Grace KG, Hastings GZ, Brown F, Rowlands DJ, Francis MJ. Presentation and immunogenicity of viral epitopes on the surface of hybrid hepatitis B virus core particles produced in bacteria. J Gen Virol. 1990;71:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Lobaina Y, García D, Abreu N, Muzio V, Aguilar JC. Mucosal immunogenicity of the hepatitis B core antigen. Biochem Biophys Res Commun. 2003;300:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Sirard JC, Niedergang F, Kraehenbuhl JP. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol Rev. 1999;171:5-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Curtiss R, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035-3043. [PubMed] |