Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1825
Revised: September 3, 2004
Accepted: October 26, 2004
Published online: March 28, 2005
AIM: To investigate effects of ischemic pre-conditioning on the liver endogenous oxidant-antioxidant system during ischemia/reperfusion injury.
METHODS: Twenty-four male Sprague-Dawley rats were randomly divided into sham-operated (Sham), ischemia/reperfusion (I/R), ischemic pre-conditioning plus ischemia/reperfusion (IPC) groups. Serum ALT, AST and hyaluronic acid levels were assayed and pathologic alterations observed. Liver malondialdehyde (MDA) contents, endogenous antioxidant enzymes, superoxidase dismutase (SOD), catalase (CAT), gultathionine peroxidase (GSH-Px) activities, neutrophils accumulation marker, myeloperoxidase (MPO) activities were measured respectively.
RESULTS: Compared with I/R group, sinusoidal endothelial cells as well as hepatocytes damages, as assessed biochemically and histochemically, were improved significantly in IPC group; neutrophils infiltration was also markedly reduced. In IPC group, liver peroxidation, as measured by MDA contents, was significantly decreased when compared with I/R group; endogenous antioxidant enzymes, SOD, CAT and GSH-Px activities were markedly higher than that in I/R group.
CONCLUSION: Ischemic pre-conditioning exerts protective effects on both hepatic sinusoidal endothelial cells and hepatocytes during liver I/R injury. Its mechanisms may involve dimunition of neutrophils infiltration and modulation of the imbalance of endogenous oxidant-antioxidant system in the organism.
- Citation: Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol 2005; 11(12): 1825-1828
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1825
Ischemia/reperfusion (I/R) injury is a major cause of morbidity and mortality following liver surgery and transplantation. Ischemic pre-conditioning (IPC) refers to brief episodes of ischemia followed by prolonged ischemia and reperfusion and has been shown to protect organs against ischemia/reperfusion injury[1]. Although, it was studied extensively in a variety of organs, including skeletal muscle, brain, spinal cord, kidney, intestine, heart and liver, the mechanisms of its protective effects remain unknown.
Reactive oxygen species (ROS) have been implicated in the pathogenesis of ischemia/reperfusion injury[2]. Administration of antioxidants such as glutathione, quercetin, could afford protection against ischemia/reperfusion injury[3,4]. It has been shown that ischemic pre-conditioning can prevent the formation of ROS after I/R, thus exerting protection to the liver[5,6]. To control the detrimental effects of ROS, organisms have developed a variety of antioxidant defense systems, especially the endogenous antioxidant enzymes system including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). However, there is little information available revealing the effects of ischemic pre-conditioning on those antioxidant enzymes. In this study, we investigated the effects of ischemic pre-conditioning on the liver endogenous oxidant-antioxidant system during ischemia/reperfusion injury.
Twenty-four male Sprague-Dawley rats, weighing 200-250 g, were obtained from the Experimental Animal Center of Wuhan University and kept under specific pathogen free conditions. The rats were randomly divided into 3 groups of 8 rats each: sham-operated (Sham) group, ischemia/reperfusion (I/R) group, ischemic preconditioning plus ischemia/reperfusion (IPC) group. Liver I/R injury was induced as follows. Rats were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). A midline laparotomy was performed. An atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. After 90 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Sham group rats underwent the same protocol without vascular occlusion. But in IPC group rats, before the procedure of ischemia/reperfusion, the left lateral and median lobes of the liver were subjected to 10 min ischemia followed by 10 min reperfusion. Three hours after liver reperfusion, all rats were sacrificed and blood and liver samples were collected for analysis.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using commercial kits by a Hitachi Automatic Biochemical Analyzer. Determination of serum hyaluronic acid was performed by radioimmunology according to the assay kit’s instructions (Shanghai Navy Medical Institute, China).
Rat livers were homogenized in 0.05 mol/L phosphate buffer. The homogenates were centrifuged at 3500 r/min for 30 min at 4 °C and the supernatant was taken for the assays of MDA and antioxidant enzymes activities (all assay kits purchased from Nanjing Jiancheng Bioengineering Co. Ltd, China).
MDA was assayed by the measurement of thiobarbituric acid-reactive substances (TBARS) levels at 532 nm. Results were expressed as nmol/mg protein.
SOD activity was measured through the inhibition of nitroblue tetrazolium (NBT) reduction by O2- generated by the xanthine/xanthine oxidase system. One SOD activity unit was defined as the enzyme amount causing 50% inhibition in 1 mL reaction solution per milligram tissue protein and the result expressed as U/mg protein.
CAT activity was determined by measuring the intensity of a yellow complex formed by molybdate and H2O2 at 405 nm, after ammonium molybdate was added to terminate the H2O2 degradation reaction catalyzed by CAT. An enzyme activity unit was defined as the degradation of 1 μmol H2O2 per sec per mg tissue protein and the enzyme activity expressed as U/mg protein.
GSH-Px activity was detected by measuring the reduction of glutathione (GSH) per min on the base of its catalysis. GSH reacts with 5,5’dithiobis-p-nitrobenzoic acid (DTNB), and produces yellow colored compounds which are detected at 412 nm and represents the reduction of GSH. One unit of the enzyme activity was defined as a decrease of 1 μmol/L GSH per min for 1 mg tissue protein after the decrease of GSH of the nonenzymatic reaction is subtracted and the result expressed as U/mg protein.
Detailed procedures for the above measurements were performed according to the kits’ protocol. The protein content was determined by the technique of Lowry et al[7], using bovine serum albumin as the standard.
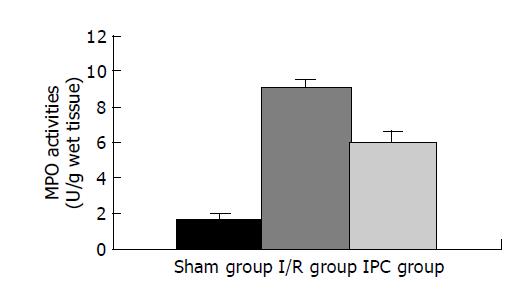
Liver samples were weighed and homogenized in a solution prepared from the assay kit (Nanjing Jiancheng Bioengineering Co. Ltd, China), and homogenates of 5% were obtained and used for MPO assay. MPO can catalyze the redox reaction of H2O2 and 3, 3, 5, 5-tetramethylbenzidine and produce yellow- colored compounds, through whose absorbance at 460 nm MPO activity was calculated and expressed as U/g wet tissue. One unit of MPO activity was defined as the quantity of enzyme that degraded 1 μmoL H2O2 at 37 °C per g wet tissue.
Liver specimens, with approximate size 1.0 cm×0.5 cm×0.3 cm, were fixed in 10% formaldehyde for 12-24 h, dehydrated gradually in a graded series of ethanol, clarified in xylene and embedded in paraffin wax. Paraffin wax sections of 5 μm were stained with hematoxylin and eosin for pathologic examination.
Data were expressed as mean±SD. The statistical significance of differences between groups were analyzed using the one-way analysis of variance (ANOVA). The P values less than 0.05 was considered statistically significant.
RESULTS
After 90 min of hepatic ischemia and 3 h of reperfusion, serum ALT and AST levels were significantly increased in I/R group, as compared with Sham group (P<0.01). However, in IPC group, serum ALT and AST levels were markedly reduced when compared with I/R group (P<0.01).
Liver levels of MDA, a marker of oxidative stress[8], were significantly higher in I/R group compared with Sham group (P<0.01). However, the MDA levels in IPC group were markedly decreased as compared with I/R group (P<0.01).
Activities of liver antioxidant enzymes, SOD, CAT and GSH-Px were presented in Table 2. In I/R group, all these enzymes activities were significantly lower compared with Sham group (P<0.01). But in IPC group, the antioxidant enzymes activities were markedly higher when compared with I/R group (P<0.01).
Neutrophil deposition at 3 h of reperfusion in the ischemic lobes, as analyzed by MPO activities[9], increased significantly in I/R group compared with Sham group (P<0.01). In contrast, IPC group had markedly reduced MPO activities as compared with I/R group (P<0.01).
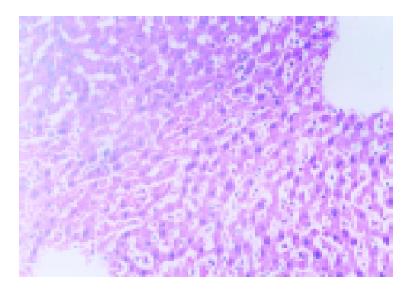
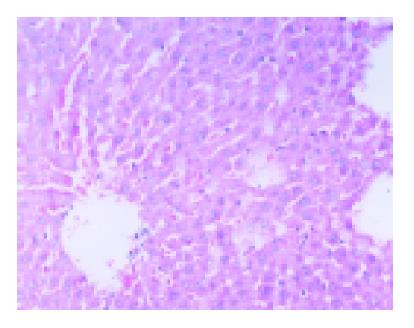
The ischemic lobes in I/R group revealed disorderly liver sinusoids, enlarged and congested with many red blood- cells, their lining endothelial cells degenerated, necrotized and sloughed off, exposing parenchymal cells immediately to blood, as well as multiple and extensive ballooning/hepatocellular necrosis and massive infiltration of neutriphils (Figure 2). However, IPC group showed good preservation of lobular architecture, with less sinusoidal lining endothelial cells swelled, necrotized and sloughed off and few hepatocellular necrosis/ballooning and neutrophils infiltration as well (Figure 3).
Hepatic ischemia/reperfusion can lead to liver cells (i.e., parenchymal and sinusoidal cells) damage and dysfunction. Ischemic pre-conditioning is extensively documented to reduce I/R injury in a variety of organs including liver[10-12]. In our study, we demonstrated that IPC could attenuate hepatic I/R injury, indicated by reduced serum ALT and AST levels and improved tissue pathologic alteration as compared with I/R group. Neutrophils contribute to the hepatic I/R injury[13]. Intravital video microscopy observed that ischemic pre-conditioning could attenuate the recruitment of leukocytes in terminal hepatic venules after liver I/R[14]. Our study also showed that IPC significantly blunted the increase of liver MPO contents, a marker of neutrophils accumulation, compared with I/R group. Hyaluronic acid (HA) is produced mainly by fibroblast and other specialized connective tissue cells, and removed from circulation by specific receptors present in sinusoidal cells (SEC) of the liver. HA uptake reflects the damage of SEC and serum HA levels were used as a noninvasive indicator of SEC damage[15,16]. Our experiment showed that the increase of serum HA levels was markedly prevented in IPC group as compared with I/R group and sinusoidal endothelial pathological alteration also significantly improved, suggesting that IPC was not limited to parenchymal cells but ameliorated sinusoidal cells dysfunction during I/R injury.
ROS is involved in the I/R injury. Liver contents of MDA, the product of lipid peroxidation, were significantly increased following I/R compared with Sham group. In IPC group, the MDA contents were markedly lowered compared with I/R group. The results are in agreement with the reports of Cavalieri et al[5]. Peralta et al[6], showed that IPC could block the xanthine oxidase pathway of ROS generation, thus providing protection against liver I/R injury. Diminishing ROS production contributes to the mechanism of IPC injury-preventive effects. To reduce the detrimental effects of ROS, besides diminishing its production, organisms have developed their own antioxidant mechanisms including low-molecular-weight antioxidant molecules, i.e., glutathiones, melatonin and various antioxidant enzymes, such as SOD, CAT, GPx and glutathione reductase. These enzymes activities are higher in the liver than in other tissues. SOD catalyses the dismutation of the superoxide anion (O2-) into H2O2; H2O2 can be transformed into H2O and O2 by CAT; GSH-Px is a selenoprotein, which reduces lipidic or nonlipidic hydroperoxides as well as H2O2 while oxidizing GSH[17]. In our study, we found that I/R impaired these enzymes activities, as indicated by the markedly lower activities compared with Sham group. But in IPC group, the decrease of enzymes activities was significantly suppressed during I/R injury. These data indicate that IPC may confer protection to the liver during I/R injury in part by improving activities of the endogenous antioxidant enzymes, which scavenge ROS and reduce their effects. However, the mechanism by which it stimulates these antioxidant enzymes is unknown. Many evidences have shown that the expressions of all these enzymes genes are directly or indirectly regulated by redox-sensitive transcription factors, such as NF-κB and AP-1[18,19]. Hepatic IPC is associated with activation of NF-kappaB[20], which may account for its stimulation of the endogenous antioxidant enzymes.
In summary, the present study showed that IPC could protect sinusoidal endothelial cells as well as hepatocytes during liver I/R injury and its mechanism is partly involved in modulating the imbalance of endogenous oxidant-antioxidant system in the organism. This may suggest a potential role for antioxidant enzymes in the management of I/R injury, but further studies are needed for their injury-preventive effects.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Rauen U, Viebahn R, Lauchart W, de Groot H. The potential role of reactive oxygen species in liver ischemia/reperfusion injury following liver surgery. Hepatogastroenterology. 1994;41:333-336. [PubMed] |
| 3. | Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864-870. [PubMed] |
| 4. | Su JF, Guo CJ, Wei JY, Yang JJ, Jiang YG, Li YF. Protection against hepatic ischemia-reperfusion injury in rats by oral pretreatment with quercetin. Biomed Environ Sci. 2003;16:1-8. [PubMed] |
| 5. | Cavalieri B, Perrelli MG, Aragno M, Mastrocola R, Corvetti G, Durazzo M, Poli G, Cutrìn JC. Ischemic preconditioning attenuates the oxidant-dependent mechanisms of reperfusion cell damage and death in rat liver. Liver Transpl. 2002;8:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Peralta C, Bulbena O, Xaus C, Prats N, Cutrin JC, Poli G, Gelpi E, Roselló-Catafau J. Ischemic preconditioning: a defense mechanism against the reactive oxygen species generated after hepatic ischemia reperfusion. Transplantation. 2002;73:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 8. | Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2421] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 9. | Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 765] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | Peralta C, Serafin A, Fernández-Zabalegui L, Wu ZY, Roselló-Catafau J. Liver ischemic preconditioning: a new strategy for the prevention of ischemia-reperfusion injury. Transplant Proc. 2003;35:1800-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fraunberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Langdale LA, Flaherty LC, Liggitt HD, Harlan JM, Rice CL, Winn RK. Neutrophils contribute to hepatic ischemia-reperfusion injury by a CD18-independent mechanism. J Leukoc Biol. 1993;53:511-517. [PubMed] |
| 14. | Sawaya DE, Brown M, Minardi A, Bilton B, Burney D, Granger DN, McDonald JC, Zibari GB. The role of ischemic preconditioning in the recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion. J Surg Res. 1999;85:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Reinders ME, van Wagensveld BA, van Gulik TM, Frederiks WM, Chamuleau RA, Endert E, Klopper PJ. Hyaluronic acid uptake in the assessment of sinusoidal endothelial cell damage after cold storage and normothermic reperfusion of rat livers. Transpl Int. 1996;9:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kobayashi H, Horikoshi K, Yamataka A, Yamataka T, Okazaki T, Lane GJ, Miyano T. Hyaluronic acid: a specific prognostic indicator of hepatic damage in biliary atresia. J Pediatr Surg. 1999;34:1791-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 785] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Rojo AI, Salinas M, Martín D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324-7334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Teoh N, Dela Pena A, Farrell G. Hepatic ischemic preconditioning in mice is associated with activation of NF-kappaB, p38 kinase, and cell cycle entry. Hepatology. 2002;36:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |