Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.79
Revised: March 8, 2004
Accepted: April 5, 2004
Published online: January 7, 2005
AIM: To explore the feasibility of enhancing apoptosis-inducing effects of chemotherapeutic drugs on human gastric cancer cells by stable transfection of extrinsic Smac gene.
METHODS: After Smac gene was transferred into gastric cancer cell line MKN-45, subclone cells were obtained by persistent G418 selection. Cellular Smac gene expression was determined by RT-PCR and Western blotting. After treatment with mitomycin (MMC) as an apoptotic inducer, in vitro cell growth activities were investigated by trypan blue-staining method and MTT colorimetry. Cell apoptosis and its rates were determined by electronic microscopy, annexin V-FITC and propidium iodide staining flow cytometry. Cellular caspase-3 protein expression and its activities were assayed by Western blotting and colorimetry.
RESULTS: When compared with MKN-45 cells, the selected subclone cell line MKN-45/Smac had significantly higher Smac mRNA (3.12±0.21 vs 0.82±0.14, t = 7.52, P<0.01) and protein levels (4.02±0.24 vs 0.98±0.11, t = 8.32, P<0.01). After treatment with 10 μg/mL MMC for 6-24 h, growth inhibition rate of MKN-45/Smac (15.8±1.2-54.8±2.9%) was significantly higher than that of MKN-45 (5.8±0.4- 24.0±1.5%, t = 6.42, P<0.01). Partial MKN-45/Smac cancer cells presented characteristic morphological changes of apoptosis under the electronic microscope with an apoptosis rate of 36.4±2.1%, which was significantly higher than that of MKN-45 (15.2±0.8%, t = 9.25, P<0.01). Compared with MKN-45, caspase-3 expression levels in MKN-45/Smac were improved significantly (3.39±0.42 vs 0.96±0.14, t = 8.63, P<0.01), while its activities were 3.25 times as many as those of MKN-45 (0.364±0.010 vs 0.112±0.007, t = 6.34, P<0.01).
CONCLUSION: Stable transfection of extrinsic Smac gene and its over-expression in gastric cancer cell line can significantly enhance cellular caspase-3 expression and activities, ameliorate apoptosis-inducing effects of mitomycin C on cancer cells, which is a novel strategy to improve chemotherapeutic effects on gastric cancer.
- Citation: Zheng LD, Tong QS, Wang L, Liu J, Qian W. Stable transfection of extrinsic Smac gene enhances apoptosis-inducing effects of chemotherapeutic drugs on gastric cancer cells. World J Gastroenterol 2005; 11(1): 79-83
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/79.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.79
Up to now, chemotherapy is still the important adjuvant treatment for postoperative and advanced gastric cancer. Its research focuses on how to reduce the side effects of chemotherapeutic drugs and improve the sensitivities of tumor cells to them[1-4]. A series of researches indicate chemotherapeutic drugs, radiotherapy and thermotherapy could all induce apoptosis to various extents by exerting their anti-tumor effects[5-7]. The second mitochondria-derived activator of caspases (Smac) or DIABLO gene is a recently identified and novel proapoptotic molecule, which is released from mitochondria into the cytosol when cell apoptosis undergoes, to enhance activities of caspase-3 through eliminating the functions of inhibitors of apoptosis proteins (IAPs)[8]. It was reported that the apoptosis-inducing effects of chemotherapeutic drugs on ovary cancer and leukemia could be significantly enhanced by gene transfer of Smac into cancer cells[9,10]. In this study, we investigated the effects of stable transfection of extrinsic Smac gene on apoptosis of gastric cancer cells induced by chemotherapeutic drugs, to explore a novel strategy to improve chemotherapeutic effects on gastric cancers.
Eukaryotic vector pcDNA3.1-Smac containing a full-length human Smac cDNA (719 bp) was kindly provided by Professor Xiao-Dong Wang (USA). Blank vector pcDNA3.1 was preserved by our central laboratory. Polyclonal rabbit anti-human Smac was a kind gift from Professor Emma (Ireland). Monoclonal mouse anti-human caspase-3 and its activity detection kit were purchased from Santa Cruz Biotechnology and Clontech Company respectively. Annexin V-FITC reagent kit was purchased from Jingmei Biotech Company. Liposome GeneSHUTTLE-40 was purchased from Q-bio Gene Limited Company. Newborn calf serum, RPMI 1640, TrizolTM reagent kit and G418 were all purchased from Gibco Company. Dimethyl sulfoxide (DMSO) and 3, [4,5- dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were both purchased from Clontech Company. Mitomycin C (MMC) was a product from Japan Union Ferment Industry Company, which was prepared into 1 g/L stock solution with PBS, preserved at 4 °C and kept from light.
Human gastric cancer cell line MKN-45 was kindly provided by Professor Ji-Hua Dong, Department of Virology, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, China. Cells were cultured in RPMI 1640 medium supplemented with penicillin/streptomycin (100 units/mL and 100 μg/mL respectively) and 10% neonatal bovine serum at 37 °C in a humidified atmosphere of 50 mL/L CO2 and passaged every three days.
The MKN-45 cells at exponential phases of growth were inoculated into a 24-well plate. At the same time, non-transfection, pcDNA3.1 and pcDNA3.1-Smac transfection groups were designed for this experiment. Gene transfection was conducted according to the protocol of liposome GeneSHUTTLE-40 (G40). In brief, 4-6 μL G40 was mixed with 1 μg pcDNA3.1-Smac or pcDNA3.1 and the mixture was incubated for 30 min, then DNA/liposome complex solutions were added into the wells. After incubated at 37 °C in a mosphere containing 5 mL/L CO2 for 48 h, the cells were digested with 0.01%EDTA, then seeded into a 6-well plate (35 mm in diameter) at 1×108/L density, and then selected with 600 mg/L G418 for 2 wk. When most of the non-transfected cells were dead, the concentration of G418 was decreased to 300 mg/L and maintained for another 2 wk. After cellular clones were formed, subclones were chosen at random and amplified. The subclone cells expressing Smac and neo genes were named as MKN-45/Smac and MKN-45/neo respectively.
Cellular Smac mRNA expression levels were assayed by reverse transcription polymerase chain reaction (RT-PCR). Total cellular RNA extraction was conducted with TrizolTM reagent kit, according to the protocol of the manufacturer. The reverse transcription was conducted at 42 °C for 30 min in 25 μL total volume containing 2 μg template RNA, 1 μL 10 mmol/L dNTP, 20 U RNasin, 1 μL Oligo dT18, 200 U AMV, 5 μL 5×AMV buffer. The PCR primers for Smac gene were designed by Primer 5.0 software: upstream 5’-CTGTGACGATTGGCTTTG-3’, downstream 5’-GTGATT CCTGGCGGTTAT-3’, which were synthesized by Shanghai GeneBase Company. The anticipated product length was 425 bp. α-tubulin (310 bp) served as an internal control. Touchdown PCR (TD PCR) was used for amplification reaction. Amplified products were separated with 2% agarose electrophoresis. The brightness ratio between Smac and α-tubulin was evaluated with a gel computer image system (MGIAS-1000, Bio-Rad Company).
Cellular Smac protein expression levels were assayed by Western blotting. The extraction, quantification and separation of proteins were conducted as previously described in Molecular Cloning. Blots were incubated sequentially with 1% nonfat dry milk, rabbit polyclonal anti-Smac antibody and goat radish peroxidase-conjugated immunoglobulin G, and evaluated using an ECL Western blotting kit. Smac protein band intensities were determined densitometrically using the video imaging CMIASWIN system.
MKN-45, MKN-45/neo and MKN-45/Smac cells were seeded at 2×108/L density into the 24-well chamber slides (each group had five wells). After cells were attached to the wells, 10 mg/L MMC was added into each well. Then cells were incubated with RPMI 1640 at 37 °Cin at mospbere containg 5 mL/L CO2 for 0 h, 6 h, 12 h, 18 h and 24 h respectively. Then cells were digested by 0.125% trypsinase + 0.01% EDTA, stained with trypan blue and counted under an inversion microscope. For each well, cell count was repeated 3 times to draw the cell growth curve.
MTT colorimetry was used. MKN-45, MKN-45/neo and MKN-45/Smac cells were seeded at 3×104/L density into 96-well chamber slides. For each cell line, untreated control group, 0.1 mg/L MMC group, 1 mg/L MMC and 10 mg/L MMC group were designed, with each group having five wells. After treating with MMC for 24 h, 20 μL MTT (5 g/L) was added into each well and cultured for another 4 h, the supernatant was discarded, then 100 μL DMSO was added. When the crystals were dissolved, the optical density A values of the slides were read on the enzyme-labeled minireader II at the wavelength of 490 nm. Cell proliferation inhibitory rate (%) = (1 - average A490nm value of experimental group/average A490nm value of control group) ×100%. For each detection, the total procedure was repeated 3 times.
After treated with 10 mg/L MMC for 24 h, three kinds of cancer cells were collected, sequentially rinsed in PBS, fixed with 2.5% glutaraldehyde for 30 min, and then washed with PBS. After routine embedment and section, cells were observed under an electron microscope.
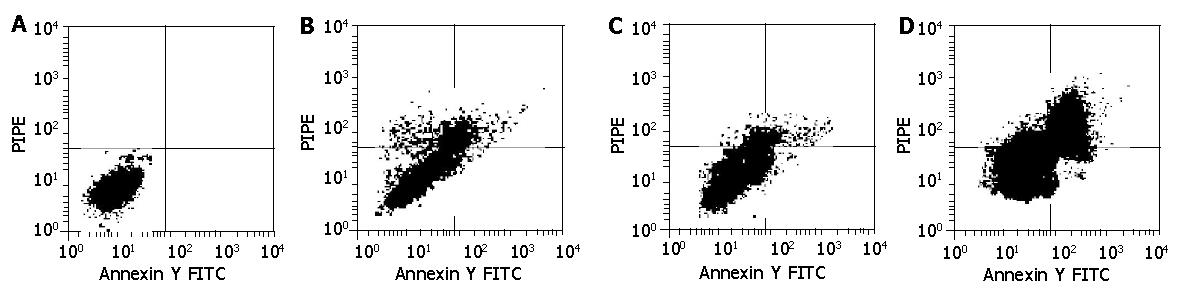
After treatment with 10 mg/L MMC for 24 h, apoptotic ratios of three kinds of cells were determinated by annexin V-FITC and propidium iodide staining flow cytometry. Cells from the above groups were collected, washed twice with cold PBS, resuspended with 100 μL binding buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 2.5 mmol/L CaCl2, pH 7.4) into 2 - 5×105 cells /mL density, and incubated with annexin V-FITC at room temperature for 10 min. After washing with binding buffer, the cells were resuspended with 400 μL binding buffer containing 10 μL propidium iodide (20 μg/mL), and incubated on ice for 15 min. Apoptosis was analyzed by flow cytometry (BD Company, USA) at a wavelength of 488 nm.
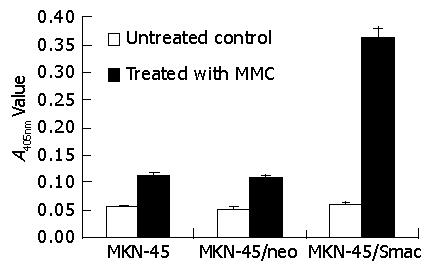
After treatment with 10 μg/mL MMC for 24 h, cellular caspase-3 protein expression levels were assayed by Western blotting (as the same method above). The caspase-3 expression levels were analyzed with the computer imaging system. 2×105 cells from above groups were respectively collected, added into 50 μL cellular lysis buffer, then incubated on ice for 10 min. After centrifugation (12000 r/min) at 4 °C for 3 min, the supernatant was collected and added sequentially into 50 μL 2×reaction buffer, 5 μL 1.0 mmol/L caspase-3 substrate DEVD-pNA, and incubated at 37 °C for 1 h. After transferred into 96 wells, the optical density A values of the slides were read on the enzyme-labeled minireader II at the wavelength of 405 nm (A405nm), which stood for the relative activities of caspase-3.
Data was expressed as mean±SD and analyzed using SPSS10.0 statistical software.
All the untransfected MKN-45 cells were dead after G418 (600 μg/mL) selection for 1 wk. The pcDNA3.1 and pcDNA3.1-Smac transfected cells were continuously selected with G418 for 4 wk, until magnificent clones could be observed. The clones were respectively amplified. The subclone MKN-45/neo and MKN-45/Smac cells were obtained, stably expressing neo and Smac genes respectively.
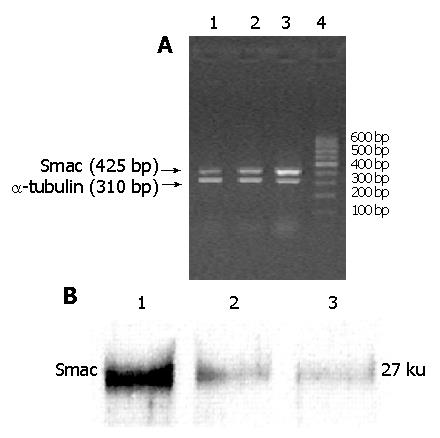
As shown in Figure 1A, after electrophoresis of RT-PCR products, Smac (425 bp) amplification bands could be observed in MKN-45, MKN-45/neo and MKN-45/Smac cells. There were only weak bands in MKN-45 and MKN-45/neo cells, and brighter bands in MKN-45/Smac cells, amplified with the same amount of RNA template. MGIAS-1000 gel computer image system proved that the Smac/α-tubulin ratio in MKN-45/Smac was 3.8 times, 3.7 times as many as that of MKN-45 (3.12±0.21 vs 0.82±0.14, t = 7.52, P<0.01), MKN-45/neo (3.12±0.21 vs 0.84±0.11, t = 8.26, P<0.01) respectively. The brightness of Smac bands between MKN-45 and MKN-45/neo had no significant difference (P>0.05).
As shown in Figure 1B,Mr27000 protein bands could be detected by Western blotting in MKN-45, MKN-45/neo and MKN-45/Smac cells. Computer image system indicated that Smac protein band brightness of MKN-45/Smac cells was 4.1 times and 4.2 times as many as that of MKN-45 (4.02±0.24 vs 0.98±0.11, t = 8.32, P<0.01) and MKN-45/neo (4.02±0.24 vs 0.96±0.13, t = 8.84, P<0.01) respectively. There was no significant difference in Smac protein band brightness between MKN-45 and MKN-45/neo cells (P>0.05).
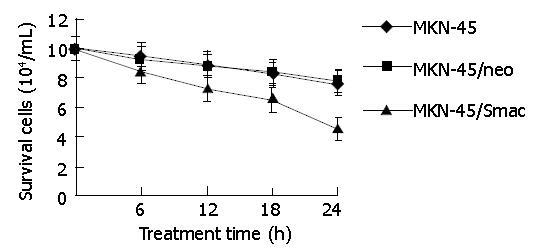
After treated with 10 mg/L MMC for 6-24 h, the in vitro growth activities of MKN-45, MKN-45/neo and MKN-45/Smac cells were all decreased. The growth inhibitory rates were 5.8±0.4-24.0±1.5%, 7.1±0.6-26.8±1.2% and 15.8±1.2-54.8±2.9% respectively. The differences in growth activities between MKN-45 and MKN-45/neo cells were not significant (P>0.05), while the growth activities of MKN-45/Smac cells were reduced by 10.0±0.9-30.8±1.5% (t = 6.42, P<0.01), when compared with those of MKN-45 cells (Figure 2).
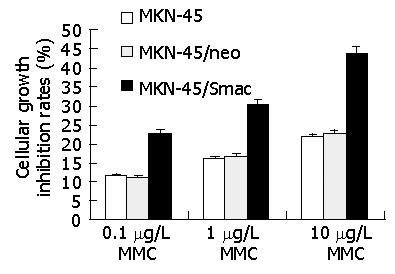
After treated with 0.1 mg/L, 1 mg/L, 10 mg/L MMC, the growth activities of MKN-45, MKN-45/neo and MKN-45/Smac cells were reduced in a time- and dose-dependent manner. After treatment with 10 mg/L MMC for 24 h, the inhibitory rate of MKN-45 cells was 21.85±1.64%, while that of MKN-45/Smac cells reached 43.71±3.12%, and the difference between these two groups was significant (t = 7.56, P<0.01). The difference in growth inhibitory rate of MMC between MKN-45 and MKN-45/neo cells was not significant (P>0.05) (Figure 3).
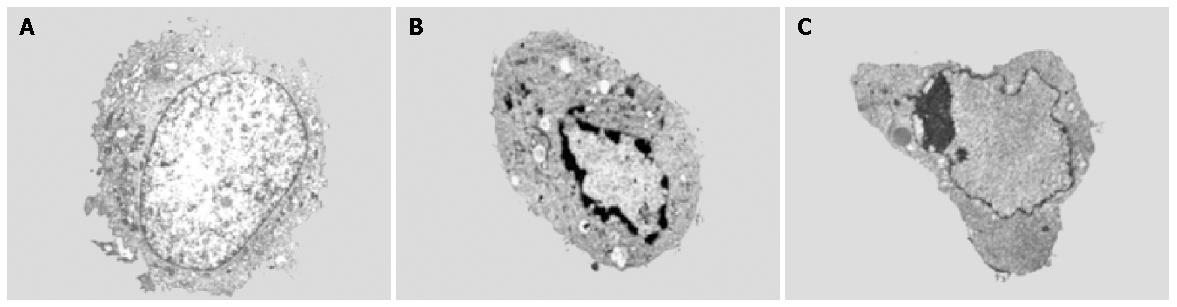
After treating with 10 mg/L MMC for 24 h, some cells had characteristic morphological changes of apoptosis under an electron microscope, such as cellular volume reduction, nuclear shrinkage, chromatin congregation around the nuclear membrane and integrity of cellular membranes (Figure 4).
After treated with 10 mg/L MMC for 24 h, the apoptotic rates of MKN-45 and MKN-45/neo cells were 15.2±0.8% and 16.5±1.1% respectively, with no significant difference (P>0.05). The apoptotic MKN-45/Smac cells increased and the apoptotic rate reached 36.4±2.1%, with a significant difference compared to that of MKN-45 (t = 9.25, P<0.01) and MKN-45/neo (t = 7.72, P<0.01) (Figure 5).
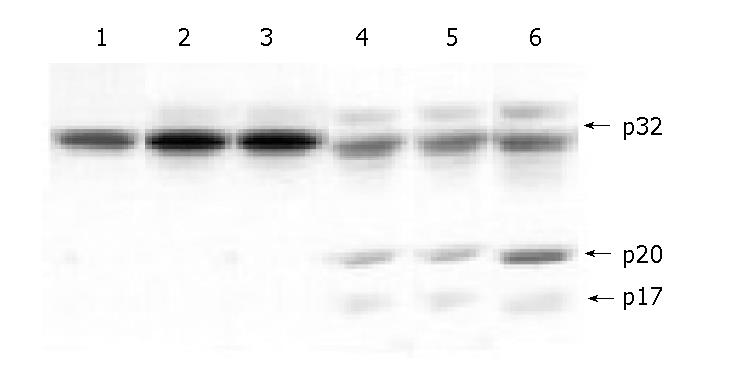
As shown in Figure 6, after treated with 10 mg/L MMC for 24 h, 17 ku (p17) and 20 ku (p20) protein bands, subunits of caspase-3, could all be detected in MKN-45, MKN-45/neo and MKN-45/Smac cells. Computer image system indicated that there was a significantly higher p20 expression in MKN-45/Smac compared to MKN-45 (3.39±0.42 vs 0.96±0.14, t = 8.63, P<0.01) and MKN-45/neo (3.39±0.42 vs 0.94±0.11, t = 9.43, P<0.01). There was no significant difference in p20 expression levels between MKN-45 and MKN-45/neo cells (P>0.05). The A405nm values of MKN-45, MKN-45/neo and MKN-45/Smac cells were 0.055±0.008, 0.052±0.012 and 0.060±0.011 respectively, while differences among them were not significant (P>0.05). After treatment with 10 μg/mL MMC for 24 h, the A405nm value reached 0.364±0.010 in MKN-45/Smac cells, which was 3.25 times as many as that in MKN-45 cells (0.112±0.007, t = 6.34, P<0.01) (Figure 7).
Gastric cancer is one of the most common malignant neoplasms in alimentary tract. Because of the side effects of chemotherapy on normal cells and drug resistance of tumor cells, it has been a research focus on how to ameliorate the chemotherapeutic effects on gastric cancer[11]. Recent researches indicate that abnormal blockage of apoptosis is an important factor for the occurrence and development of cancer[12-14]. To understand apoptosis mechanisms is hopeful for improving the sensitivities of tumor cells to chemotherapeutic drugs, and overcoming drug resistance[15,16].
The mechanisms of apoptosis are highly conserved in all sorts of species, including a series of processes. Apoptosis usually has three phases: initiation, effectors and execution[17-19].
When external stimuli induce cell apoptosis by different pathways, such as death receptor-mediated and stress-dependent pathways, imbalance between activators and inhibitors of apoptosis occurs, thus activating caspase family and changing mitochondrial outer membrane permeability, finally resulting in cell apoptosis[20]. Mitochondria are regarded as the key regulation element of cell death and the target of many proapoptotic signal pathways[21,22].
Some researches demonstrate that there are inhibitors of apoptosis proteins (IAPs) in mammalian cells, which suppress apoptosis by inhibiting procaspase activation and catalytic activity of mature caspases[23,24]. IAPs, including MIHA (mammalian IAP homolog A, or called XIAP), c-IAP1, c-IAP2 and survivin, could bind directly to caspase-3, caspase-7 and caspase-9, and inhibit their activities, which are the downstream effectors during cascades of caspase family. MIHA could also suppress apoptosis induced by chemotherapeutic agents, UV-irradiation and Bax[25-27]. It is suggested that cellular IAPs accumulation is one of the reasons for cancer cells to escape the killing effects of anti-cancer agents[28].
Smac or DIABLO gene is a proapoptotic molecule, which is released from mitochondria into the cytosol, along with cytochrome C (cyt-C) during apoptosis. As an important apoptotic modulator, Smac functions as eliminating the caspase-inhibitory properties of IAPs[29]. Some researches have found that Smac could promote apoptosis via two pathways, which are dependent on the interaction between Smac and IAPs[30]. One hydrolyzes caspase-3 protein, and the other enhances the catalytic activity of mature caspase-3. McNeish et al[9] constructed the adenoviral vector for Smac gene, and transfected it into ovarian cancer cells. They found that apoptotic cancer cells increased remarkably, and cell apoptosis mediated by Smac was not dependent on cyt-C and Bcl-2, but realized through Caspase-9. Jia et al[10] stably transfected full-length (FL) and mature (MT) Smac genes into K562 and CEM leukemic cell lines, and they concluded that both FL and MT Smac transfection could promote the sensitivity of leukemic cells to UV light, and activate cellular caspase-9 and caspase-3.
In this study, an extrinsic Smac gene was transfected into gastric cancer cells which induced its over-expression. We found that Smac overexpression could enhance the apoptosis-inducing effects of MMC, by electronic microscopy and annexin V-FITC and propidium iodide staining flow cytometry. These results are consistent with those recently reported by Guo et al[31] (2002) and Fulda et al[32] (2002), in which Smac could enhance apoptosis in leukemia and malignant neuroglioma cells induced by chemistry or immunology in vivo. Western blotting and colorimetry were sequentially used to assay the cellular caspase-3 protein expression and its activities, and it was found that stable transfection of an extrinsic Smac gene could increase the cellular activity levels of caspase-3 after treating with MMC, which accords with the functional mechanisms of Smac. These results provide a novel strategy to improve chemotherapeutic sensitivity in gastric caner patients and reduce their side effects, thus establishing a basis for further exploring the roles of Smac gene in apoptosis regulation of gastric cancer.
Assistant Editor Guo SY Edited by Zhang JZ and Wang XL
| 1. | Van Cutsem E, Haller D, Ohtsu A. The role of chemotherapy in the current treatment of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Janunger KG, Hafström L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg. 2002;168:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol. 2002;8:1023-1028. [PubMed] |
| 4. | Yao JC, Ajani JA. Adjuvant and preoperative chemotherapy for gastric cancer. Curr Oncol Rep. 2002;4:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1698] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 6. | Szostak MJ, Kyprianou N. Radiation-induced apoptosis: predictive and therapeutic significance in radiotherapy of prostate cancer (review). Oncol Rep. 2000;7:699-706. [PubMed] |
| 7. | Schulze PC, Adams V, Busert C, Bettag M, Kahn T, Schober R. Effects of laser-induced thermotherapy (LITT) on proliferation and apoptosis of glioma cells in rat brain transplantation tumors. Lasers Surg Med. 2002;30:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2467] [Cited by in RCA: 2443] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 9. | McNeish IA, Bell S, McKay T, Tenev T, Marani M, Lemoine NR. Expression of Smac/DIABLO in ovarian carcinoma cells induces apoptosis via a caspase-9-mediated pathway. Exp Cell Res. 2003;286:186-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Jia L, Patwari Y, Kelsey SM, Srinivasula SM, Agrawal SG, Alnemri ES, Newland AC. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene. 2003;22:1589-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Marcus SG, Cohen D, Lin K, Wong K, Thompson S, Rothberger A, Potmesil M, Hiotis S, Newman E. Complications of gastrectomy following CPT-11-based neoadjuvant chemotherapy for gastric cancer. J Gastrointest Surg. 2003;7:1015-1022; discussion 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer--implications for therapy. Semin Oncol. 2004;31:90-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Brown JM, Wilson G. Apoptosis genes and resistance to cancer therapy: what does the experimental and clinical data tell us? Cancer Biol Ther. 2003;2:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Ther. 2003;2:573-580. [PubMed] |
| 15. | Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Sun SY, Hail N, Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004;96:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 386] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Derradji H, Baatout S. Apoptosis: a mechanism of cell suicide. In Vivo. 2003;17:185-192. [PubMed] |
| 19. | Debatin KM. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 2004;53:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1305] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 21. | Gulbins E, Dreschers S, Bock J. Role of mitochondria in apoptosis. Exp Physiol. 2003;88:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Hockenbery DM, Giedt CD, O'Neill JW, Manion MK, Banker DE. Mitochondria and apoptosis: new therapeutic targets. Adv Cancer Res. 2002;85:203-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Martin SJ. Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell. 2002;109:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568-8580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 318] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Lotocki G, Keane RW. Inhibitors of apoptosis proteins in injury and disease. IUBMB Life. 2002;54:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Vaziri SA, Grabowski DR, Tabata M, Holmes KA, Sterk J, Takigawa N, Bukowski RM, Ganapathi MK, Ganapathi R. c-IAP1 is overexpressed in HL-60 cells selected for doxorubicin resistance: effects on etoposide-induced apoptosis. Anticancer Res. 2003;23:3657-3661. [PubMed] |
| 27. | Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-8589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 686] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 28. | Notarbartolo M, Cervello M, Dusonchet L, Cusimano A, D'Alessandro N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002;180:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Verhagen AM, Vaux DL. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2002;7:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Vaux DL, Silke J. Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun. 2003;304:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Guo F, Nimmanapalli R, Paranawithana S, Wittman S, Griffin D, Bali P, O'Bryan E, Fumero C, Wang HG, Bhalla K. Ectopic overexpression of second mitochondria-derived activator of caspases (Smac/DIABLO) or cotreatment with N-terminus of Smac/DIABLO peptide potentiates epothilone B derivative-(BMS 247550) and Apo-2L/TRAIL-induced apoptosis. Blood. 2002;99:3419-3426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (1)] |