Published online Jan 7, 2005. doi: 10.3748/wjg.v11.i1.73
Revised: March 21, 2004
Accepted: April 5, 2004
Published online: January 7, 2005
AIM: Heat shock protein (HSP)70 is over-expressed in human gastric cancer and plays an important role in the progression of this cancer. We investigated the effects of antisense HSP70 oligomer on human gastric cancer cell line SGC-7901, and its potential role in gene therapy for this cancer.
METHODS: Human gastric cancer cell line SGC-7901 was treated in vitro with various concentrations of antisense HSP70 oligonucleotides at different intervals. Growth inhibition was determined as percentage by trypan blue dye exclusion test. Extracted DNA was electrophoresed on agarose gel, and distribution of cell cycle and kinetics of apoptosis induction were analyzed by propidium iodide DNA incorporation using flow cytometry, which was also used to detect the effects of antisense oligomer pretreatment on the subsequent apoptosis induced by heat shock in SGC-7901 cells. Proteins were extracted for simultaneous measurement of HSP70 expression level by SDS-PAGE Western blotting.
RESULTS: The number of viable cells decreased in a dose- and time-dependent manner, and ladder-like patterns of DNA fragments were observed in SGC-7901 cells treated with antisense HSP70 oligomers at a concentration of 10 μmol/L for 48 h or 8 μmol/L for 72 h, which were consistent with inter-nucleosomal DNA fragmentation. Flow cytometric analysis showed a dose- and time-dependent increase in apoptotic rate by HSP70 antisense oligomers. This response was accompanied with a decrease in the percentage of cells in the G1 and S phases of the cell cycle, suggesting inhibition of cell proliferation. In addition, flow cytometry also showed that pretreatment of SGC-7901 cells with HSP70 antisense oligomers enhanced the subsequent apoptosis induced by heat shock treatment. Western blotting demonstrated that HSP70 antisense oligomers inhibited HSP70 expression, which preceded apoptosis, and HSP70 was undetectable at the concentration of 10 μmol/L for 48 h or 8 μmol/L for 72 h.
CONCLUSION: Antisense HSP70 oligomers can abrogate HSP70 expression in SGC-7901 cells, which may in turn induce apoptosis and inhibit cell proliferation, conversely suggesting that HSP70 is required for the proliferation and survival of human gastric cancer cells under normal conditions.
- Citation: Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World J Gastroenterol 2005; 11(1): 73-78
- URL: https://www.wjgnet.com/1007-9327/full/v11/i1/73.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i1.73
Gastric cancer is one of the most common malignant tumors in the world[1-3]. Although surgery and chemotherapy are effective for patients with localized tumors, the prognosis of patients with advanced or metastatic tumors is not ideal[4-6]. Therefore, it is absolutely necessary to explore a novel modality of treatment. Heat shock proteins (HSPs) or stress proteins are molecular chaperones that are induced by various environmental and pathophysiological stimuli[7], of which the Mr 70000 heat shock protein (HSP70) has been shown to be not only required for the maturation of proteins in cell growth under normal conditions, but also involved in the regulation of cell growth and transformation[8,9]. It has been reported that various malignant tumors over-express HSP70, which closely relates to tumorigenesis, malignant phenotype, tumor immunity, resistance to apoptosis and a poor prognosis in the clinical course[9-13]. Up to now, many studies have demonstrated that HSP70 is over-expressed in human gastric cancer and may contribute to the development and prognosis of this cancer[14-18]. Guo et al[15] reported that over-expression of HSP70 protein in human gastric cancer might play an important role in promoting cell growth and inhibiting apoptosis. Thus, it is conceivable that the specific inhibition of HSP70 expression may affect the proliferation and survival of human gastric cancer cells.
Antisense oligonucleotides (ON) are short stretches of nucleic acids that bind to complementary target mRNA forming mRNA-ON hybrid molecules that inhibit mRNA translation, and thereby reducing the activity of targeted gene products[19]. HSP70 antisense ON inhibit growth and induce apoptosis of human prostate cancer cells PC-3 and LNCaP[20], Molt-4 tumor cells[21], human oral squamous carcinoma cell HSC-2[9], monoblastoid U937 and murine fibrosarcoma WEHI-S cells[13], and Jurkat T cells[22]. Here, we performed this study to investigate the inhibitory effects of antisense oligonucleotide targeted to human HSP70 mRNA on proliferation and survival of human gastric cancer cell line SGC-7901 under normal conditions , and its potential role in gene therapy for this cancer.
Fifteen-mer nuclease-resistant phosphorothiolate oligodeo-xynucleotides (antisense and sense) were synthesized and purified in Shanghai Shenggong Biological Engineering Corporation (Shanghai, China). HSP70 antisense oligomer (5’-CGCGGCTTTGGCCAT-3’) was complementary to the initiation codon and 4 downstream codons of human HSP70 mRNA[23]. The corresponding sense oligomer (5’-ATGGCCAAAGCCGCG-3’) was used as control. In this study, we did not utilize drug delivery systems, such as liposomes or vector transfection, to allow the antisense ON molecules to gain access to the cells. Because our previous results of the kinetic studies of HSP70 oligodeoxynucleotide metabolism in human prostate cancer PC-3m cells have shown that nuclease-resistant phosphorothiolate oligodeoxynucleotides (antisense and sense) could be directly taken up by PC-3m cells through endocytosis within 90-180 min and exist stably for over 24 h inside the cells, and that these oligomers have a high specificity to bind to the correspondent HSP70 mRNA of the cells (unpublished data). Similarly, Saikawa et al[24] also showed that the effectiveness of drug delivery simply by means of spontaneous uptake of cyclin D1 antisense ON could increase resistance to endogenous nucleases.
Cell culture Human gastric cancer cell line SGC-7901 used in this study was obtained from Shanghai Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and maintained in RPMI 1640 medium supplemented with 100 mL/L fetal calf serum (FCS), 100 kU/L penicillin, 100 mg/L streptomycin and 2 mmol/L L-glutamine in a humidified incubator containing 50 mL/L CO2 at 37 °C.
Antisense oligonucleotide treatment Exponentially growing SGC-7901 cells at 1×109/L in culture were treated with HSP70 antisense or sense oligonucleotides at the concentrations of 1, 2, 4, 6, 10, 12, 14 and 16 μmol/L for 48 h, or 8 μmol/L for 24, 48, 72, 96 and 100 h. The culture medium was changed every 24 h by fresh RPMI 1640 medium, containing the same concentration of HSP70 antisense or sense oligonucleotides. The control cultures were left untreated at 37 °C for the same period of time.
Analysis of cell proliferation inhibition The number of viable cells was determined by trypan blue dye exclusion test, and the percentage of cell proliferation inhibition was calculated by the following formula: Inhibition % = (N-NT)/(N-NO) ×100%, where N is the number of untreated cells cultured for n d, NO is the cell number on d 0, and nt is the number of treated cells cultured for n d[21].
Heat-shock treatment SGC-7901 cells were first treated with 10 μmol/L HSP70 antisense and sense oligomers, respectively for 24 h, then harvested and suspended at 5×108/L in closed Eppendorf tubes (1.5 mL). The closed tubes were left in a water bath at 42 °C for 2 h, centrifuged at 1000 r/min for 5 min and then the cells were re-suspended in fresh medium containing HSP70 antisense and sense oligomers of the same dose before returning to a 37 °C incubator for an additional 24 h. Also, SGC-7901 cells were treated in parallel with antisense or sense oligomers or heat shock alone. The percentage of hypodiploid/apoptotic cells was calculated by flow cytometry.
Flow cytometry Apoptotic cells were identified and quantitated as the percentage of cells with hypodiploid DNA as assessed by propidium iodide (PI) incorporation. After treating as described above, the cells were harvested and treated with RNase and centrifuged at 1000 r/min for 10 min. The cell pellet was gently resuspended in 1 mL of hypotonic fluorochrome solution (PI 50 mg/L in 1 g/L sodium citrate plus 1 mmol/L Tris, 0.1 mmol/L EDTA and 1 mL/L Triton X-100). After 30 min at 4 °C in the dark, the cells were washed with cold phosphate-buffered-saline (PBS), then analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif) with cell fit software. The data were registered as a logarithmic scale. The forward scatter (FSC) and side scatter (SSC) of particles were simultaneously measured. Cell debris were excluded from analysis by approximately raising the FSC threshold. At least 10000 cells of each sample were analyzed. All measurements were done using the same instrument settings. Apoptotic cells were observed in the cell-cycle distribution. Cell-cycle analysis was also simultaneously performed.
Agarose gel DNA electrophoresis The pattern of DNA fragmentation was analyzed by agarose gel electrophoresis. A total of 3×106 SGC-7901 cells were lysed with lysis buffer containing 50 mmol/L Tris/HCI (pH 8.0), 2.5 mL/L NP40 and 10 mmol/L EDTA. RNase A was added at a final concentration of 200 mg/L and incubated for 1 h at 37 °C. Thereafter, the cells were treated with proteinase K (300 μg/mL) and incubated for an additional hour at 37 °C. After 4 μL of loading buffer was added, 20 μL of extracted DNA samples in each lane was electrophoresed on 15 g/L agarose gel at 50 V for 2 h and stained with ethidium bromide (EB).
Western blot The specific inhibition of HSP70 expression in SGC-7901 cells by HSP70 antisense oligomers was analyzed by Western blotting. A total of 2×106 SGC-7901 cells were lysed in lysis buffer. The samples were denatured in sample buffer, sodium dodecylsul (SDS) and resolved on 100 g/L polyacrylamide (PAGE). After electrophoresis, the proteins were separated by SDS/PAGE gels and then transferred to nitrocellulose membranes by electroblotting. The membrane blots were rinsed with 20 mmol/L Tris, 500 mmol/L NaCL, 0.5 mL/L Tween-20 (pH 7.5) and blocked by 30 g/L defatted milk. The blots were probed first with anti-HSP70 monoclonal antibodies (mAb) (Dako, Glostrup, Denmark) for 2 h. After washed in 30 g/L defatted milk, biotinylated second antibodies (Dako, Glostrup, Denmark) were incubated for an additional hour. Then the blots were transferred to Vectastain ABC. 3,3’-diaminobenzidine (DAB) substrate kits for horseradish peroxidase were used to develop the color of bands.
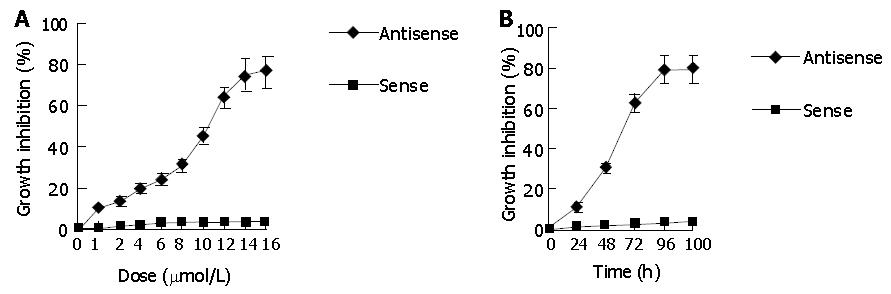
Treatment of SGC-7901 cells with HSP70 antisense oligomers resulted in inhibition of cell proliferation. The inhibiting proliferation rate of SGC-7901 cells increased with the treatment dose and incubation time of HSP70 antisense oligomers, which was observed at 1 μmol/L and reached maximum at the concentrations of 14 μmol/L for 48 h or 8 μmol/L for 96 h (Figure 1). Moreover, the number of died cells increased as the dose reached 16 μmol/L for 48 h and 8 μmol/L for 100 h. These effects were not observed in the cells treated with sense oligomers. These results indicated that HSP70 antisense treatment of SGC-7901 cells not only inhibited cell proliferation but also induced cell death in a dose- and time-dependent manner.
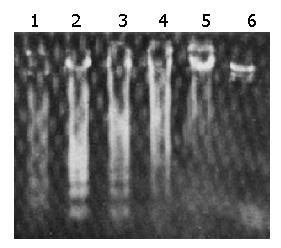
Apoptosis-characteristic ladder-like patterns of DNA fragments consisting of approximately 180-200 bp on 15 g/L agarose gel electrophoresis were observed in SGC-7901 cells treated with HSP70 antisense oligomers at a concentration of 10 μmol/L for 48 h or 8 μmol/L for 72 h, which were consistent with inter-nucleosomal DNA fragmentation (Figure 2). When the dose reached over 16 μmol/L for 48 h and 8 μmol/L for 100 h, the smearing patterns of DNA electrophoresis were observed (Figure 2), indicating DNA fragments from cell death, which were consistent with the result from trypan blue dye exclusion test. In contrast, ladder-like patterns of DNA fragments were not found in SGC-7901 cells treated the same doses of sense oligomers and the same incubation time as antisense oligomers. It confirmed that HSP70 antisense oligomer treatment could induce apoptosis of SGC-7901 cells.
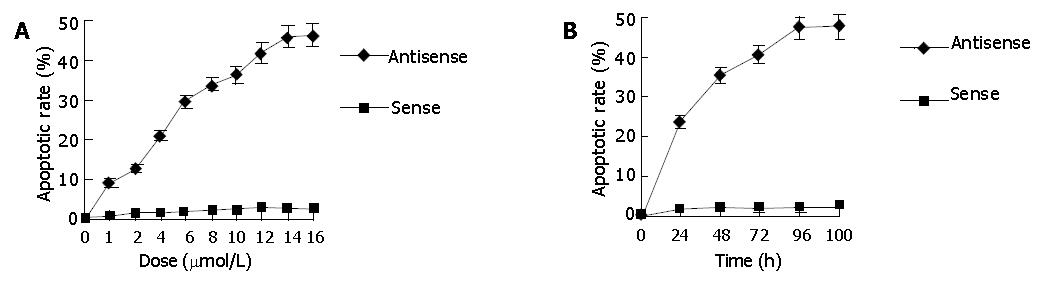
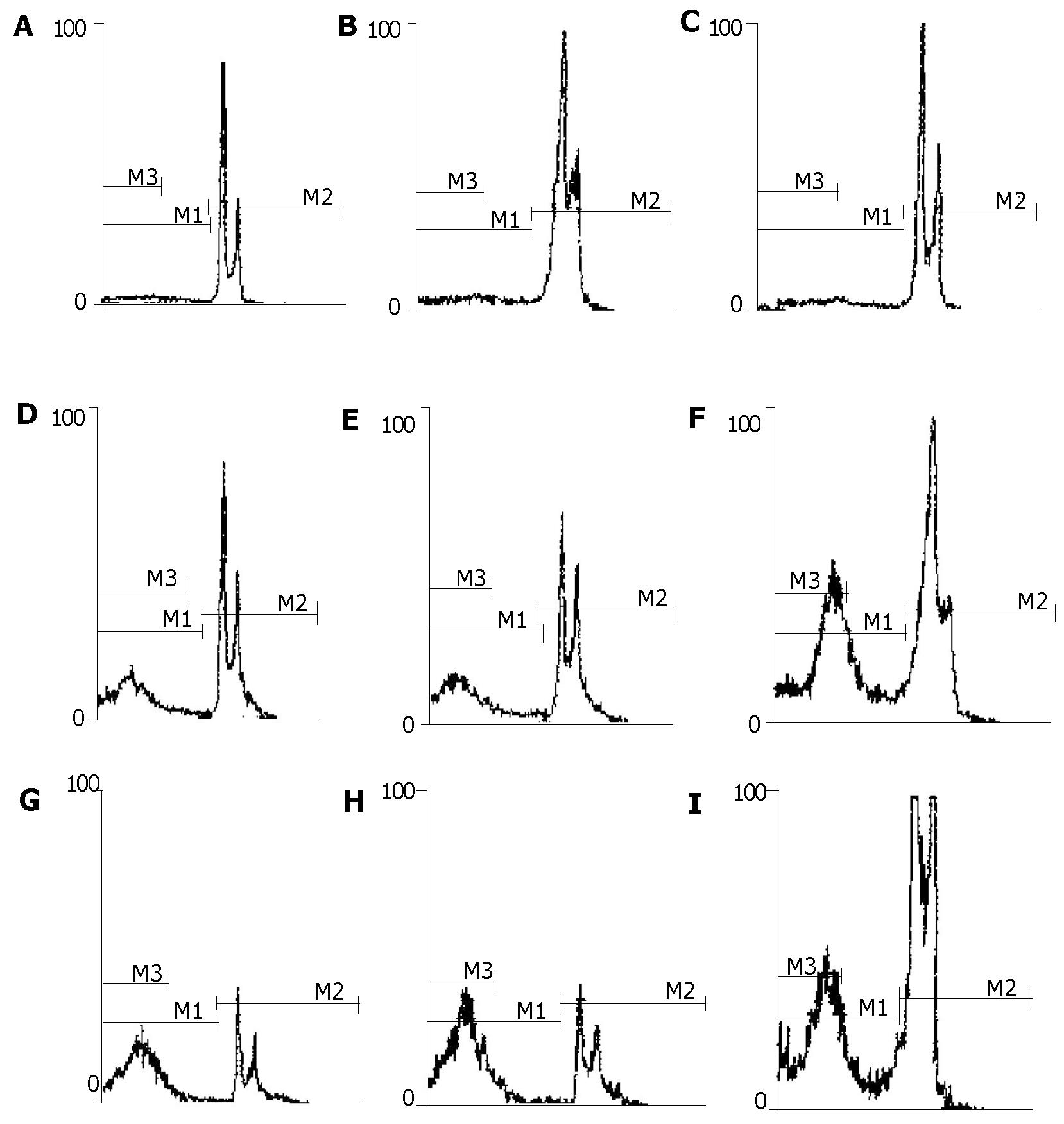
The distribution of SGC-7901 cells varied in different cell cycles as analyzed by flow cytometry. The representative flow cytograms displaying cell cycle specificity distribution of untreated cells and those treated with antisense and sense oligomers are shown in Figure 4. The DNA histograms contained G0/G1 peak, S-phase region and G2 + M peak in SGC-7901 cells treated with HSP70 sense oligomers as well as untreated cells, while the cells treated with HSP70 antisense oligomers showed an extra peak of DNA content, i.e., a peak of hypodiploid cell population. In other words, apoptotic cells were found in HSP70 antisense oligomer-treated SGC-7901 cells. There was a significant decrease in the percentage of G1 and S-phases among the total SGC-7901 cells when the number of hypodiploid/apoptotic cells increased with the elevation of HSP70 antisense oligomer dose and the prolongation of incubation time intervals (Figure 4). Also, kinetic analysis by flow cytometry showed that the apoptotic rate of SGC-7901 cells induced by HSP70 antisense oligomers was dependent on their doses and incubation time intervals, which was observed at 1 μmol/L for 48 h and reached maximum at 14 μmol/L for 48 h or 8 μmol/L for 96 h. Under the same conditions, all the effects above were not observed in SGC-7901 cells treated with HSP70 sense oligomers (Figure 3). Thus, the results further confirmed that HSP70 antisense oligomers could induce apoptosis of SGC-7901 cells in a dose- and time-dependent manner, and it seemed most likely that the inhibition of SGC-7901 cell proliferation might in part be a result of apoptotic cell death, which mainly occurred in G1 and S-phases of cell cycle.
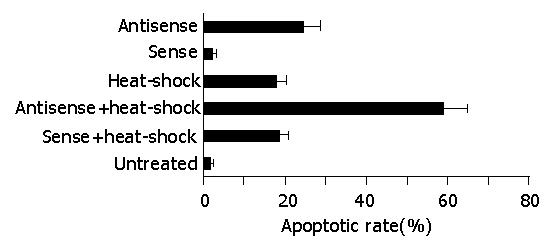
Since HSP70 plays an important role in protecting cells from injury or repairing damage imposed by heat shock, and heat shock itself induces apoptosis, we further investigated whether the abrogation of HSP70 by antisense oligomer treatment could enhance the induction of apoptosis by heat shock. Flow cytometric analysis revealed that the percent of apoptotic cells induced by heat shock was significantly higher in antisense-oligomer-treated cells than in untreated cells, i.e., treatment with HSP70 antisense oligomers resulted in an increase in the number of hypodiploid/apoptotic cells in response to heat shock (Figure 5). In contrast, HSP70 sense oligomers had no effect. Taken together, the results indicated that HSP70 antisense oligomer treatment could induce apoptosis not only in normally growing SGC-7901 cells, but also in heat-stressed cells.
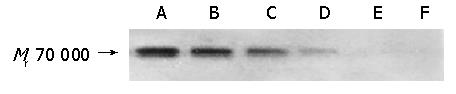
Western blot was performed to confirm the specific inhibition of HSP70 expression by antisense HSP70 oligomers (Figure 6). The results showed that treatment of SGC-7901 cells with 8 μmol/L HSP70 antisense oligomer for 48 and 72 h or with 10 μmol/L for 48 h resulted in the inhibition of HSP70 expression (Figure 6), while treatment with 10 μmol/L sense HSP70 oligomer for 48 h or 8 μmol/L for 72 h had no effect on HSP70 protein expression (Figure 6).
The role of antisense ON against human telomerase RNA[25,26], cyclin D1[24,27], bcl-2[28], her-2/neu (c-erbB-2) gene[29], and epidermal growth factor receptor (EGFR)[30] in gastric cancer has been reported. However, to our knowledge, the effects of antisense HSP70 oligomers on gastric cancer cells have not been investigated as yet. The present study demonstrated that HSP70 antisense oligomers could induce apoptosis and inhibit the growth of human gastric cancer SGC-7901 cells. SGC-7901 cells treated with HSP70 antisense oligomers at the concentration of 10 μmol/L for 48 h or 8 μmol/L for 72 h displayed the abrogation of HSP70, ladder-like patterns of DNA fragments which were consistent with internucleosomal DNA fragmentation, and a hypodiploid DNA peak of propidium iodide-stained nuclei analyzed by flow cytometry, characteristics of an apoptotic mode of cell death. When the dose reached over 16 μmol/L for 48 h and 8 μmol/L for 100 h, the cells died significantly, which was comfirmed by DNA electrophoresis (Figure 2), suggesting that antisense HSP70 treatment not only inhibits proliferation, but also induces death of SGC-7901 cells. By flow cytometric analysis of propidium iodide-stained cells and quantitating of the subdiploid apoptotic peak, we observed that HSP70 antisense oligomers could induce apoptosis of SGC-7901 cells in a dose- and time-dependent manner, which strongly correlated with the results from classical DNA fragmentation assays in agarose gel electrophoresis. Furthermore, HSP70 antisense oligomers were found to elevate the number of hypodiploid cells, i.e., apoptotic cells reduced the number of cells at the G1 and S phases without affecting the total cell number. Thus, antisense HSP70 oligomer treatment may cause apoptosis mainly in cells at the G1 and S phases of a cell cycle, and inhibit cell proliferation. The apoptosis- inducing and growth- inhibiting effects of HSP70 antisense oligomers are dose- and time-dependent. The HSP70 gene is constitutively expressed at G1/S boundary and in S phase of a cell cycle, and HSP70 protein is necessary for cells to enter into the early S phase during proliferation[31]. A G1-specific enhancer, HSP-MYCB sequence, has been identified in human HSP70 gene, which is responsible for the cell cycle-dependent expression of HSP70[32]. Moreover, DNA sequence-specific inhibition of HSP70 expression by HSP70 antisense oligomers in SGC-7901 cells precedes apoptosis of these cells. The findings of our study can confirm the previous reports on induction of apoptosis and inhibition of proliferation by abrogation of HSP70 expression in other kinds of tumor cells[9,13,21,22], indicating the inhibitory effects of antisense HSP70 oligomers in cancer cells. In addition, the decreased expression of HSP70 due to antisense oligomers can enhance the induction of apoptosis by heat shock. This effect was also observed in Molt-4 cells by Wei et al[21]. In the present study, it seemed likely that HSP70 antisense oligomer treatment could inhibit HSP70 expression of SGC-7901 cells, which in turn could induce apoptosis in both normal and heat-stressed cells and inhibit cell proliferation. The inhibition of cell proliferation shown here may, in part, be a result of cell death induced by apoptosis. The results also suggest that HSP70 might not be a mere marker of biological stress in tumor cells, but is essential for the proliferation and survival of human gastric cancer cells under normal conditions. These findings may be of importance in searching for new agents for human gastric cancer therapy by inhibiting or blocking HSP70 synthesis.
Although the mechanism of anti-apoptotic effect of HSP70 remains obscure, some studies have shown that decreasing HSP70 expression levels in tumor cells can induce cell apoptosis and inhibit tumor growth[9,21]. Tumor cells having low HSP70 levels have been shown to respond to apoptotic stimuli by activation of stress-activated protein kinases, generation of free radicals, early disruption of mitochondrial transmembrane potential, release of cytochrome-C from the mitochondria and activation of Caspase-3-like proteases, suggesting that HSP70 rescues cells from apoptosis in the death signaling pathway[33]. The mechanism of HSP70 antisense oligomer in inducing cell apoptosis and inhibiting cell growth, is unclear. In view of HSP70 as a molecular chaperone playing an important role in protein metabolisms, such as folding, assembly, disassembly and degradation, as well as in protection against various environmental stresses, it is speculated that HSP70 antisense oligomer sequence can specifically cross-link with HSP70 mRNA and block HSP70 gene expression, thus specifically inhibiting synthesis of HSP70 at the transcription level in the G1 and S phases of a cell cycle, which may render tumor cells unable to maintain normal proliferation or activate the signal transduction pathway of apoptosis, leading to cell apoptosis and inhibition of cell growth.
In summary, HSP70 as a molecular chaperone plays an important role in cell proliferation, survival and modulation of apoptosis in human gastric cancer cells under normal conditions. HSP70 antisense oligomers can specifically inhibit proliferation of gastric cancer cell line SGC-7901 by inducing apoptosis through cell cycle arrest following HSP70 abrogation. Thus, antisense HSP70 nucleotide strategy may be a promising approach to human gastric cancer therapy.
Edited by Kumar M and Wang XL
| 1. | Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649-651. |
| 2. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 3. | Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. hTERT expression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508-512. |
| 4. | Maehara Y, Kakeji Y, Oda S, Takahashi I, Akazawa K, Sugimachi K. Time trends of surgical treatment and the prognosis for Japanese patients with gastric cancer. Br J Cancer. 2000;83:986-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Borie F, Millat B, Fingerhut A, Hay JM, Fagniez PL, De Saxce B. Lymphatic involvement in early gastric cancer: prevalence and prognosis in France. Arch Surg. 2000;135:1218-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Cascinu S, Graziano F, Barni S, Labianca R, Comella G, Casaretti R, Frontini L, Catalano V, Baldelli AM, Catalano G. A phase II study of sequential chemotherapy with docetaxel after the weekly PELF regimen in advanced gastric cancer. A report from the Italian group for the study of digestive tract cancer. Br J Cancer. 2001;84:470-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Ashburner M, Bonner JJ. The induction of gene activity in drosophilia by heat shock. Cell. 1979;17:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 984] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 8. | Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063-1081. [PubMed] |
| 9. | Kaur J, Kaur J, Ralhan R. Induction of apoptosis by abrogation of HSP70 expression in human oral cancer cells. Int J Cancer. 2000;85:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Maehara Y, Oki E, Abe T, Tokunaga E, Shibahara K, Kakeji Y, Sugimachi K. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Witkin SS. Heat shock protein expression and immunity: relevance to gynecologic oncology. Eur J Gynaecol Oncol. 2001;22:249-256. [PubMed] |
| 13. | Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 382] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Cheng SB, Hong JQ, Wang YH. Expression and significance of heat shock protein 70 and p53 protein in gastric carcinoma. Shiyong Aizheng Zazhi. 2000;15:241-242. |
| 15. | Guo JC, Zhang XY, Yang YS, Ye L, Li JC, Fan DM. Overexpression of heat shock protein (HSP70) in human gastric cancer. Aizheng. 1999;18:45-46. |
| 16. | Isomoto H, Oka M, Yano Y, Kanazawa Y, Soda H, Terada R, Yasutake T, Nakayama T, Shikuwa S, Takeshima F. Expression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancer. Cancer Lett. 2003;198:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Maehara Y, Oki E, Abe T, Tokunaga E, Shibahara K, Kakeji Y, Sugimachi K. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144-151. |
| 18. | Canöz O, Belenli O, Patiroglu TE. General features of gastric carcinomas and comparison of HSP70 and NK cell immunoreactivity with prognostic factors. Pathol Oncol Res. 2002;8:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wagner RW. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994;372:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 563] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 20. | Gibbons NB, Watson RW, Coffey RN, Brady HP, Fitzpatrick JM. Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. Prostate. 2000;45:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Wei YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother. 1995;40:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Liossis SN, Ding XZ, Kiang JG, Tsokos GC. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol. 1997;158:5668-5675. [PubMed] |
| 23. | Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455-6459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 607] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 24. | Saikawa Y, Kubota T, Otani Y, Kitajima M, Modlin IM. Cyclin D1 antisense oligonucleotide inhibits cell growth stimulated by epidermal growth factor and induces apoptosis of gastric cancer cells. Jpn J Cancer Res. 2001;92:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Yang SM, Fang DC, Yang JL, Liang GP, Lu R, Luo YH, Liu WW. Effect of antisense human telomerase RNA on malignant phenotypes of gastric carcinoma. J Gastroenterol Hepatol. 2002;17:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Wong SC, Yu H, Moochhala SM, So JB. Antisense telomerase induced cell growth inhibition, cell cycle arrest and telomerase activity down-regulation in gastric and colon cancer cells. Anticancer Res. 2003;23:465-469. [PubMed] |
| 27. | Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y, Fan DM. Antisense to cyclin D1 reverses the transformed phenotype of human gastric cancer cells. World J Gastroenterol. 1999;5:18-21. [PubMed] |
| 28. | Wacheck V, Heere-Ress E, Halaschek-Wiener J, Lucas T, Meyer H, Eichler HG, Jansen B. Bcl-2 antisense oligonucleotides chemosensitize human gastric cancer in a SCID mouse xenotransplantation model. J Mol Med (Berl). 2001;79:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Funato T, Kozawa K, Fujimaki S, Miura T, Kaku M. Increased sensitivity to cisplatin in gastric cancer by antisense inhibition of the her-2/neu (c-erbB-2) gene. Chemotherapy. 2001;47:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Hirao T, Sawada H, Koyama F, Watanabe A, Yamada Y, Sakaguchi T, Tatsumi M, Fujimoto H, Emoto K, Narikiyo M. Antisense epidermal growth factor receptor delivered by adenoviral vector blocks tumor growth in human gastric cancer. Cancer Gene Ther. 1999;6:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Milarski KL, Morimoto RI. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci USA. 1986;83:9517-9521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 227] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Taira T, Narita T, Iguchi-Ariga SM, Ariga H. A novel G1-specific enhancer identified in the human heat shock protein 70 gene. Nucleic Acids Res. 1997;25:1975-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124-6134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 522] [Article Influence: 19.3] [Reference Citation Analysis (0)] |