Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1246
Revised: January 1, 2004
Accepted: January 15, 2004
Published online: May 1, 2004
AIM: To examine the role of nucleostemin in the growth regulation of gastric cancer, liver cancer and other cancers.
METHODS: RT-PCR was used to clone the fragment of nucleostemin cDNA from HEK 293 cells. Eighteen kinds of malignant tumor tissues including gastric adenocarcinoma and liver cancer tissues, 3 kinds of benign tumor tissues, 3 kinds of benign hyperplastic tissues and normal tissues were employed to examine nucleostemin gene expression by RT-PCR, Slot blot, Northern blot and in situ hybridization.
RESULTS: We successfully cloned a 570 bp fragment of nucleostemin-cDNA from HEK-293 cells. All detected malignant tumor tissues, benign tumor tissues, and benign hyperplastic tissues had high levels of nucleostemin expression. Nucleostemin was also expressed in human placenta tissue at a high level. In terminally differentiated normal human adult kidney and mammary gland tissues, no nucleostemin expression could be detected.
CONCLUSION: Nucleostemin can help regulate the proliferation of both cancer cells and stem cells. It might play an important role in the growth regulation of gastric cancer, liver cancer and other cancers.
- Citation: Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ, Hu GF, Wei YY, Lao WD. Role of nucleostemin in growth regulation of gastric cancer, liver cancer and other malignancies. World J Gastroenterol 2004; 10(9): 1246-1249
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1246
Perhaps the most important and useful property of stem cells is self-renewal. Through this property, striking parallels can be found between stem cells and cancer cells: tumor may originate from the transformation of normal stem cells. Similar signaling pathways may regulate self-renewal in stem cells, and cancer cells[1]. McKay and Tsai found a novel gene nucleostemin (NS) in rat embryo stem cells, the rat central nervous system (CNS) stem cells and rat primitive bone marrow cells. NS gene was apparently involved in regulating the proliferation of both stem cells and at least some types of cancer cells[2]. The protein encoded by NS was abundantly expressed while the cells were proliferating in an early multipotential state, but it abruptly and almost entirely disappeared at the start of differentiation. The fact that NS expressed in stem cells and several cancer cell lines, but not in the differentiated cells of adult tissues, suggested its role in maintaining self-renewal of stem cells and cancer cells. Our recent data also showed that NS expressed in human gastric cancer (SGC-7901) cells, human hepatocarcinoma (HepG2) cells, human cervical cancer (Hela) cells, human osteosarcoma (OS-732) cells, human mammary (MMK-7) cells and human embryo kidney (HEK-293) cells[3]. Coordinated control of self-renewal and commitment to differentiation is key to maintaining the homeostasis of the stem cell compartment[4,5], when deregulated it may contribute to cancer pathogenesis[6,7]. The identification of stem-cell-specific proteins and the elucidation of novel regulatory pathways that ensure the integration of these processes, are therefore of fundamental importance[8,9]. It seems that NS is involved in the regulatory pathways, but the fundamentals about NS are still unknown. For example, why does NS express in stem cells and cancer cells but not in differentiated cells? What are the tumor types that misexpress NS and the underlying mechanism? And what is the timing of NS reactivation in cancer cells.
Gastric cancer is common in China, and its early diagnosis and treatment are difficult[10,11]. To investigate the mechanism of carcinogenesis of gastric cancer would facilitate its diagnosis and treatment. Therefore, NS may take part in the growth regulation of tumor tissues and hyperplastic tissues. So we employed a number of malignant tumor tissues, benign tumor tissues, benign hyperplastic tissues and normal tissues to examine the expression of NS gene by RT-PCR, Slot blot, Northern blot and in situ hybridization, which would help illuminate the mechanism of self-renewal of cancer cells.
We collected tens of tumor tissue samples including gastric adenocarcinoma, liver cancer, bladder carcinoma, pancreatic cancer and esophagus squamous carcinoma samples. We also collected several kinds of normal tissue samples such as normal kidney tissues and muscle tissues. Human embryo kidney (HEK-293) cells were cultured in DMEM containing 100 mL/L FCS at 37 °C in 50 mL/L CO2[12,13]. The cells were kept in logarithmic growth phase by trypsin digestion and reinoculation every 2-4 d[14,15]. DMEM cell culture medium was purchased from Gibco Corporation; fetal calf serum (FCS) and trypsin from Hyclone Corporation; restriction enzymes and T4 DNA ligase from Promega Corporation. PCR primers were synthesized by Shanghai Shenyou Company and DNA sequencing was also performed by this company.
Total RNA in HEK 293 cells was isolated using total RNA isolation kit (Promega Corporation). A 2 μg total RNA was used in reverse transcription (RT) reaction. Up-stream primer, 5’-ggatccatgaaaaggcctaagttaaagaaagc (BamHI site underlined); Down-stream primer, 5’-aagcttgctctccaaattctcctttggta (HindIII site underlined). PCR protocol (94 °C 30 s→50 °C 30 s→72 °C 30 s) was run for 30 cycles and PCR product was about 570 bp. PCR product was separated by agrose gel electrophoresis, recovered with gel extraction kit (Omega Corporation), and ligated with pGEM-T vector. The ligate was transformed into competent E.coli JM 109 cells. The correct transformant was identified by restriction enzyme analysis and DNA sequencing.
About 100 mg tissues of each sample was used to isolate total RNA using total RNA isolation kit (Promega Corporation). RT protocol and PCR protocol including PCR primers are the same as the above. GAPDH was used as a loading control, its up-stream primer, 5’-ggtggacctgacctgccgtctaga, and its down-stream primer, 5’-ttactccttggaggccatgtggg. PCR protocol (94 °C 30 s→55 °C 30 s→72 °C 20 s) was run for 25 cycles.
Twenty μg total RNA of each sample was added into slots, and transferred onto nylon membrane. Slot blot analysis was carried out using 32P-labeled NS fragments and human 18S RNA as probes in the hybridization.
Equal volume of total RNA of each sample was used in denaturing agarose gel electrophoresis, and after that RNA was transferred onto nylon membrane. Northern blot analysis was made using 32P labeled NS fragments and human 18S RNA as probes in the hybridization.
Nucleostemin cDNA fragment was inserted into pGEM-T vector, and large quantities of plasmid was harvested with Plasmid Extraction Kit (Promega Corporation). After linearization by NcoI restriction enzyme, SP6 promoter was used to drive the transcription of NS-cRNA probes in vitro and in this system Biotin labeled rUTPs was introduced into NS-cRNA probes. In the same way, after linearization by Not I restriction enzyme, T7 promoter was used to drive the transcription of sense NS-cRNA probes in vitro, which acted as the negative control.
The expression of NS mRNA was detected in situ by Biotin SP-HRP method[16,17]. Pretreatment of paraffin-embedded specimens referred to the previously used methods[13]. In brief, before in situ hybridization, sections were deparaffinized in xylene (7.5 min, twice) and rehydrated in graded ethanol. Sections were then rinsed in 2 changes of diethylpyrocarbonate-treated distilled water and rinsed in 10 mmol/L citrate buffer, pH 6.0. Sections were placed in glass racks (20 slides/rack) and submerged in approximately 250 mL citrate buffer in covered glass tubes. Sections were then microwaved in a 900-watt microwave (Panasonic Matsushita Electric, Danville, MA) 3 times for 5 min each time at full power, topped up with additional citrate buffer to compensate for evaporation. Slides were allowed to cool slightly, then were rinsed in 2 changes of diethylpyrocarbonate-treated distilled water, dehydrated in graded ethanol, and dried. Hybridization of the labeled probes to the tissue sections was carried out using a previously described method[18]. Sense NS-cRNA probes and SP-HRP solution were used as negative controls. And the tissue sections were counterstained by hematoxylin.
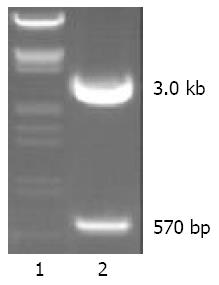
We successfully cloned a 570 nt fragment by RT-PCR in HEK-293 cells. Then the fragment was ligased with pGEM-T vector and the recombinant vector was named pGEM-T-NS. pGEM-T-NS was digested by BamH I and HindIII enzymes, yielding a 3.0 kb fragment of vector and a 570 bp fragment of NS cDNA (Figure 1). The result of sequencing indicated our sequence of NS cDNA was completely identical to the sequence in Genebank. Sequencing revealed that no base mutation occurred in the sequence of our cloned NS-cDNA fragment.
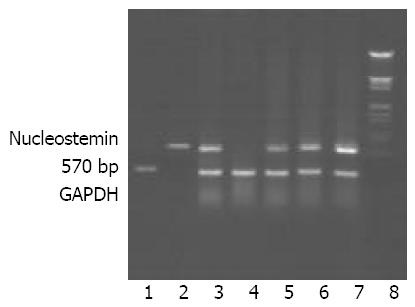
According to the results of RT-PCR, all above malignant tumor tissues, benign tumor tissues, and benign hyperplastic tissues had high levels of NS expression, so did the normal human placenta tissues. And a lower level of NS expression in normal human adult muscle tissues could also be detected. However, no NS expression could be detected in normal human adult kidney tissues and mammary gland tissues. Partial results of RT-PCR are shown in Figure 2.
Slot blot analysis indicated that NS expressed in all above malignant tumor tissues, benign tumor tissues, and benign hyperplastic tissues, which was in agreement with the results of RT-PCR. However, no NS expression could be detected in normal human adult kidney tissues, mammary gland tissues and muscle tissues. Partial results of Slot blot analysis are shown in Figure 3.
The results of Slot blot were further confirmed by Northern blot analysis. NS misexpressed in all these malignant tumor tissues, benign tumor tissues, and benign hyperplastic tissues. But the results of Northern blot analysis showed NS did not express in normal human adult kidney tissues, mammary gland tissues and muscle tissues. Partial results of Northern blot analysis are shown in Figure 4.
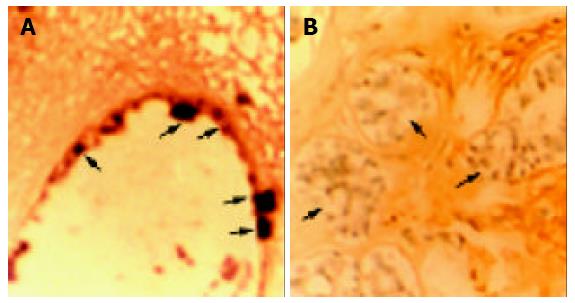
The Biotin SP-HRP (streptavidin-biotin-peroxidase-DAB) system reacted with 0.05% DAB (3-3’, 4-4’-diaminobenzidine tetrahydrochloride) containing 0.1 mL/L H2O2, producing brown positive staining. And counterstain was performed by hematoxylin, producing azury nuclear staining. The results of in situ hybridization conformed to those of Slot blot analysis and Northern blot analysis. Positive staining was located in cytoplasm of cancer cells, but no positive staining in cytoplasm of normal adult tissue cells such as normal kidney cells and mammary gland cells. The results of in situ hybridization detection of nucleostemin mRNA in invasive ductal breast carcinoma and normal mammary gland tissue are indicated in Figure 5. Ductal carcinoma cells showed strong staining in cytoplasm (Figure 5A), while normal mammary glandular epithelial cells showed no positive staining, only with azury nuclear counterstaining (Figure 5B).
Nucleostemin (NS) is a newly found p53-binding protein, which exists mainly in the nucleoli of stem cells and some various cancer cells, but does not express in committed and terminally differentiated cells[2]. The expression level of NS declined obviously during embryo and adult development due to the differentiation of stem cells. For example, it was expressed preferentially in neuroepithelial precursors during embryogenesis and diminished during the central nervous system (CNS) differentiation. The expression of NS was also found in adult bone marrow hematopoietic stem cells, however could not be found in committed B-lymphocytes and granulocytes. In vivo experiments displayed that the NS expression could not even be detected after CNS stem cells were induced to differentiate. Recently, it was confirmed that NS expression also existed in ES cells and mesenchymal stem cells of mice[19]. In vivo, NS expression disappeared before the changes of cell cycle markers during the development of CNS, which indicated the disappearance of NS expression induced the cell cycle arrest, but not the reverse. Moreover, non-cycling cells increased by silencing NS expression with small interfering RNA (siRNA) in CNS stem cells and U2OS cancer cells[2].
We successfully cloned a 570 bp fragment of NS-cDNA from HEK-293 cells. The full length of NS-cDNA was 1 650 bp and the cloned fragment was located in the 5’-terminal of the full length. The fragment had high specificity and non-homology with other genes by BLAST analysis, so it was equal to probes in the screening of gene expression. From the results of RT-PCR, Northern blot and Slot blot analysis, we found that all these malignant tumor tissues, benign tumor tissues, and benign hyperplastic tissues had high levels of NS expression, which indicated that NS played an important role in the self-renewal of these cancer cells and hyperplastic cellls. At the same time, NS expressed in human placental tissues also at a high level, possibly due to the existence of a mass of placental stem cells[20-23]. A small quantity of NS expression could be found in normal human muscle tissues by RT-PCR, however no expression could be detected by Slot blot and Northern blot analysis. The reason might be that a few of myoblasts with the characteristic of stem cells existed in muscle tissues[24,25], so only by the sensitive RT-PCR technique could a very small volume of NS expression be detected (figures not provided). In terminally differentiated normal human adult kidney tissues and mammary gland tissues, no NS expression could be detected as no cells possess the characteristics of stem cells or cancer cells in these tissues. The analysis of in situ hybridization indicated that NS gene expressed in cancer cells, but not in normal adult tissue cells, such as normal kidney cells and mammary gland cells. These results were in agreement with McKay’s[2].
All these results displayed that NS might play an important role in the proliferation regulation of cancer cells and stem cells. A working hypothesis of NS deduced from all information involved displayed that NS accumulated predominantly in the nucleolus and NS localized into nucleoplasm after binding with GTP[26]. In the nucleoplasm, NS and p53 existed in a protein complex, and subsequently the growth-suppressive function of p53 was inhibited. During cell differentiation, when NS expression was downregulated, p53 was released from the protein complex, which allowed the stabilization or activation of p53 and induction of target genes critical for cell cycle exit and differentiation, such as p21CIP. Members of the E2F family of transcription factors play a crucial role in cell proliferation control by regulating the expression of genes important for DNA synthesis and cell cycle progression[27]. A functional E2F factor is composed of a heterodimer between an E2F polypeptide (E2F1- E2F6) and a DP polypeptide (DP1 and DP2). The transcriptional activities of E2F factors are regulated through association with the Rb (retinoblastoma) tumor suppressor protein and the other pocket proteins, p107 and p130. Binding of a pocket protein inhibits the transcriptional activation capacity of E2F factors and, in at least some cases, can convert E2F to repressors of transcription. The activity of Rb is regulated during the cell cycle by cdks (cyclic dependent kinase), primarily by cdk4 and cdk6, in association with D-type cyclin and cyclin E in association with cdk2. Phosphorylation of Rb by these cdks results in the dissociation of Rb from E2F and the depression/activation of E2F-regulated genes. The E2F-DP transcription complex determined the start of S phase and induced the expression of some genes which were necessary to the progress of S phase, such as Cyclin E, Cyclin A, proliferating cell nuclear antigen (PCNA) and dihydrofolate reductase (DHFR). The product of Cip1, p21CIP1, blocked the process of phosphorylation of Rb by cdks and Cyclin complex, so E2F-DP in non-phosphorylation state still bound with RB and could not be freed. Only freed E2F-DP could gain its activity to promote the expression of these genes, and as a result, DNA synthesis was carried out and cells would pass through S phase.
Our results were in agreement with the working hypothesis mentioned above. There were high levels of NS expression in cancer cells, hyperplastic tissue cells and stem cells, so NS inhibited the p53-induced cell differentiation or apoptosis and these cells could easily pass through cell cycles. NS helped regulate the proliferation of both cancer cells and stem cells, although the precise mechanism was not yet clear. The focus on NS has potential therapeutic implications. For example, the growth of gastric cancer may be suppressed by silencing the NS expression with some molecular techniques, which would greatly improve the validity of its treatment.
We want to thank Dr. Hong Gao and Dr. Siguo Liu in our group for their helpful discussions and research suggestions. We also thank Ms. Lin Hou from pathological center of Peking University for her help with in situ hybridization experiments and Professor. Hongwei Zhang and her colleagues from Beijing Haidian Hospital for their help with the pathological analysis.
Edited by Ma JY Proofread by Xu FM
| 1. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. |
| 2. | Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991-3003. |
| 3. | Liu SJ, Cai ZW, Liu YJ, Dong MY, Zhang HW, Hu GF, Liu SG, Gao H, Zhang ZH, Liu XL. The effect of knocking-down nucleostemin gene expression on the in vitro proliferation and in vivo tumorigenesis of HeLa cells. J Exp Clin Cancer Res. 2004;23:529-538. |
| 4. | Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003;100:13350-13355. |
| 5. | Goldschmidt-Clermont PJ. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. Am Heart J. 2003;146:S5-12. |
| 6. | Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:16105-16110. |
| 7. | Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966-970. |
| 8. | Fiorini M, Ballarò C, Sala G, Falcone G, Alemà S, Segatto O. Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene. 2002;21:6530-6539. |
| 9. | Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328-2334. |
| 10. | Lu JB, Sun XB, Dai DX, Zhu SK, Chang QL, Liu SZ, Duan WJ. Epidemiology of gastroenterologic cancer in Henan Province, China. World J Gastroenterol. 2003;9:2400-2403. |
| 11. | Zhang C, Liu ZK. Gene therapy for gastric cancer: a review. World J Gastroenterol. 2003;9:2390-2394. |
| 12. | Nadeau I, Sabatié J, Koehl M, Perrier M, Kamen A. Human 293 cell metabolism in low glutamine-supplied culture: interpretation of metabolic changes through metabolic flux analysis. Metab Eng. 2000;2:277-292. |
| 13. | Liu SJ, Hu GF, Liu YJ, Liu SG, Gao H, Zhang CS, Wei YY, Xue Y, Lao WD. Effect of human osteopontin on proliferation, transmigration and expression of MMP-2 and MMP-9 in osteosarcoma cells. Chin Med J (Engl). 2004;117:235-240. |
| 14. | Christoffers AB, Kreisler M, Willershausen B. Effects of esradiol and progesterone on the proliferation of human gingival fibroblasts. Eur J Med Res. 2003;8:535-542. |
| 15. | Song J, Rolfe BE, Hayward IP, Campbell GR, Campbell JH. Effects of collagen gel configuration on behavior of vascular smooth muscle cells in vitro: association with vascular morphogenesis. In Vitro Cell Dev Biol Anim. 2000;36:600-610. |
| 16. | Palumbo A, Napolitano A, Carraturo A, Russo GL, d'Ischia M. Oxidative conversion of 6-nitrocatecholamines to nitrosating products: a possible contributory factor in nitric oxide and catecholamine neurotoxicity associated with oxidative stress and acidosis. Chem Res Toxicol. 2001;14:1296-1305. |
| 17. | Durán N, Bromberg N, Kunz A. Kinetic studies on veratryl alcohol transformation by horseradish peroxidase. J Inorg Biochem. 2001;84:279-286. |
| 18. | Busuttil BE, Frauman AG. Extrathyroidal manifestations of Graves' disease: the thyrotropin receptor is expressed in extraocular, but not cardiac, muscle tissues. J Clin Endocrinol Metab. 2001;86:2315-2319. |
| 19. | Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235-1249. |
| 20. | Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. |
| 21. | Bernatchez R, Belkacemi L, Rassart E, Daoud G, Simoneau L, Lafond J. Differential expression of membrane and soluble adenylyl cyclase isoforms in cytotrophoblast cells and syncytiotrophoblasts of human placenta. Placenta. 2003;24:648-657. |
| 22. | Dhot PS, Nair V, Swarup D, Sirohi D, Ganguli P. Cord blood stem cell banking and transplantation. Indian J Pediatr. 2003;70:989-992. |
| 23. | Alvarez-Silva M, Belo-Diabangouaya P, Salaün J, Dieterlen-Lièvre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437-5444. |
| 24. | Berendse M, Grounds MD, Lloyd CM. Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly. Exp Cell Res. 2003;291:435-450. |
| 25. | Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod Biol Endocrinol. 2003;1:101. |
| 27. | Nevins JR. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585-593. |