Published online Apr 1, 2004. doi: 10.3748/wjg.v10.i7.1056
Revised: March 3, 2003
Accepted: March 15, 2003
Published online: April 1, 2004
AIM: Our aims were to determine the normal limits of subepithelial basement membrane (SEBM) thickness in order to more accurately diagnose collagenous colitis in the population from southern Turkey and to investigate into links between SEBM thickness and age, and sex.
METHODS: The study included 100 patients (mean age 50.0 ± 13.3 years; male, 34; female, 66) with miscellaneous gastrointestinal symptoms, and normal colonic mucosal appearance in colonoscopic evaluation. Biopsies were taken from five different regions of the colon. SEBM was measured with a calibrated eyepiece on specimens prepared with specific stains for collagen. Intensity of inflammatory cells was graded semiquantitatively. Differences in SEBM thickness among the different colon regions, and relationships between SEBM thickness and age, sex, and density of inflammatory cells were statistically evaluated.
RESULTS: The cecum and rectum showed the largest amounts of infiltrate. None of the specimens showed histologic findings of collagenous colitis. The SEBM thicknesses measured for each case ranged from 3-20 μm. The biggest thickness was observed in rectal mucosa (median value: 10 μm). Cecum and ascending colon showed similar SEBM thickness (median value: 5 μm). SEBM thickness was not correlated with patient age or sex, but was positively correlated with the intensity of inflammatory cells in each colon segment.
CONCLUSION: In this patient group from southern Turkey, SEBM was thickest in the rectum. Our results indicate that, in this population, SEBM thickness is not correlated with age or sex, but is positively correlated with severity of inflammation. The findings also support the concept that measuring SEBM thickness at one segment in the colon is inadequate and may be misleading.
- Citation: Kayaselcuk F, Serin E, Gumurdulu Y, Ozer B, Tuncer I, Boyacioglu S. Subepithelial basement membrane thickness in patients with normal colonic mucosal appearance in colonoscopy: Results from southern Turkey. World J Gastroenterol 2004; 10(7): 1056-1058
- URL: https://www.wjgnet.com/1007-9327/full/v10/i7/1056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i7.1056
Lindstrom introduced the term “collagenous colitis” to describe a colon biopsy that showed hyalinized substance, known as a collagen band, between the transition zone of the subluminal basement membrane and the lamina propria[1-8]. The English literature contains approximately 200 reports of this condition[7]. Collagenous colitis is most often diagnosed in middle-aged women[3,6-10]. In some patients this condition is associated with autoimmune diseases, such as diabetes mellitus, primary biliary cirrhosis, rheumatoid arthritis, and gluten-sensitive enteropathy[3]. Some of the cases linked with histories of smoking, or antibiotic or non-steroidal anti-inflammatory drug (NSAID) ingestion[1,3,6,7,9,11].
In healthy adults, the subepithelial basement membrane (SEBM) of the colon consists of collagen types I and IV, laminin, and fibronectin. The thickness of this layer varies from 0 to 7 µm[3,5-7,12], and is slightly greater in the settings of hyperplastic polyps, colonic diverticulum, and congenital megacolon[3,6]. In this study, we investigated SEBM thickness in biopsies from five different colonic sites in patients from the southern Anatolia region of Turkey. One of our aims was to determine the normal limits of SEBM thickness in this region in order to more accurately diagnose collagenous colitis in the population. Another objective was to investigate into links between SEBM thickness and age, and sex.
The study included 100 patients who were admitted to our gastroenterology clinic with non-specific symptoms, such as chronic constipation, diarrhea, and abdominal distension and discomfort. Only two individuals had experienced short episodes of diarrhea. All the participants gave their informed consent for diagnostic colonoscopy. The protocol was carried out in accordance with the Helsinki Declaration as revised in 1989.
Each individual underwent colonoscopy and biopsies were collected from five different regions of the colon: the cecum, the ascending, transverse, and descending colon, and the rectum. Two biopsies were obtained from each segment. The specimens were fixed in 40 g/L neutral buffered formaldehyde, embedded in paraffin, and stained with hematoxylin-eosin, and slides were examined under the light microscope. Each slide was assessed for type of inflammation, intensity of inflammatory cells, superficial epithelial damage, cryptitis, and crypt abscesses. The finding of few lymphocytes, plasma cells, eosinophils, macrophages and scattered mast cells in lamina propria is considered to be normal in histopathological examination. Taken such a scene as normal (grade 0), intensity of inflammatory cells was graded on a three-tier scale. The predominant cell type (lymphocyte, eosinophil, or neutrophil) in the inflammatory infiltrate was also noted.
All the specimens were also histochemically prepared with periodic acid-Schiff (PAS), Masson’s trichrome, and crystal violet stains. A calibrated eyepiece was used to measure SEBM thickness on the sections stained with Masson’s trichrome at × 400 magnification. On every slide, we measured the thickest part of the SEBM in non-tangential areas of tissue section that were distant from the crypts. The SEBM thickness for each segment in each patient was recorded. Also, for each patient, two of the same biopsied regions were randomly selected and the average SEBM thickness for these two sites was recorded. PAS stain was used to demonstrate intestinal parasites and fungi, and crystal violet was used to reveal the presence of amyloid. Differences among SEBM thickness in the different colon regions, and relationships between SEBM thickness and age, and sex were statistically evaluated. The data of SEBM thickness are reported as medians (ranges). Comparisons between median values of SEBM thickness belonging to each colon segment were performed using Mann-Whitney U test. The relationships between SEBM thickness and demographic parameters (age and sex), and SEBM thickness and intensity of inflammation were tested with Spearman’s correlation. P values < 0.05 were considered statistically significant.
The mean age in the study group was 50.0 ± 13.3 yr (range, 18-82 yr). Of the 100 patients, 34 were men and 66 were women. In 93 of the cases, the mucosa appeared normal on colonoscopy. Of the 7 cases with abnormal findings, 3 showed mildly hyperemic mucosa, 2 exhibited colonic diverticula, 1 had a polyp, and 1 had a superficial ulcer. In the biopsy specimens, eosinophils were as numerous as lymphocytes in the inflammatory infiltrates. The cecum and rectum showed the largest amounts of infiltrate. In most cases, the inflammatory infiltrate in the rectal biopsies contained lymphocytes, plasmocytes, and neutrophils. None of the specimens showed amyloid deposition, intraepithelial lymphocytosis, epithelial vacuolization, desquamation, mucin loss, pseudostratification, or focal neutrophilic cryptitis.
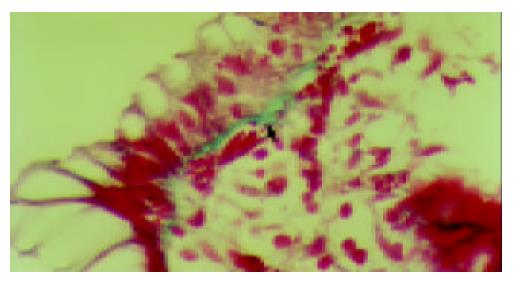
The range and median SEBM thickness in each of the five colon segments are summarized in Table 1. The median SEBM thickness for the separate segments differed. The largest thickness was observed in rectal mucosa. The difference between rectum and transverse colon did not reach statistical significance, but there was a trend towards greater thickness in rectum. Cecum and ascending colon showed similar SEBM thickness. SEBM thickness was not correlated with patients’ age or sex, but was positively correlated with intensity of inflammatory cells in each colon segment (Table 2). The thickness measured for each case ranged from 3-20 µm (Figure 1). In 1 of the 2 individuals with colonic diverticula, the SEBM in the descending colon and rectum was greater than 10 µm thick. In the 1 patient who had a polyp, the SEBM thickness in the rectum was 12 µm. In 2 cases, SEBM thickness was 15-20 µm in all segments of the colon. Both these individuals (a 50-year-old male and a 69-year-old woman) had long histories of chronic constipation and intermittent ingestion of NSAIDs. The colon biopsies in these 2 cases showed mild eosinophil infiltration.
| Colon segment | Correlation coefficient (r) | P |
| Cecum | 0.499 | < 0.0001 |
| Ascending colon | 0.642 | < 0.0001 |
| Transverse colon | 0.699 | < 0.0001 |
| Descending colon | 0.620 | < 0.0001 |
| Rectum | 0.553 | < 0.0001 |
The main histopathological criteria for collagenous colitis include the characteristic findings of a thick, continuous, hypocellular, eosinophilic, linear fibrous band beneath the surface epithelium, as well as intraepithelial lymphocytosis, epithelial vacuolization, and desquamation[1,3,5-7,11]. One of the difficulties in diagnosing collagenous colitis is that different sources cite different SEBM thickness as one of the main criteria for diagnosis[9]. Thus, sampling error is a probable cause of under diagnosis in collagenous colitis. Some investigators consider thickness > 10 µm to be diagnostic, whereas others use > 15 µm, and still others > 30 µm[3,5-7,11]. The SEBM thickness documented in cases of collagenous colitis in the English literature range from 11.5 to 60 µm[6,13-18]. In a study of 564 patients conducted in 1982, the authors identified > 10 µm in thickness as abnormal[14]. In a series of 33 patients with collagenous colitis, Tanaka et al[9] observed that 82% exhibited > 10 µm in SEBM thickness in at least one area of the left colon. The rectum appeared normal in 72% of these cases, and only three patients showed a collagen band in the cecum. Tanaka et al used < 7 µm as the criterion for normal SEBM thickness, and identified mucosal inflammation and SEBM thickness > 10 µm as diagnostic criteria for collagenous colitis[9].
SEBM thickness in the healthy population in the various studies in the literature ranges from 0 µm to 7 µm[3,6,15]. In some of these investigations, estimates of SEBM thickness in the general population were derived from autopsy series[6,14]. In a series of 457 patients that included adenocarcinoma, polyp, and megacolon cases as well as autopsy cases, Gledhill and Cole were only able to determine SEBM thickness in 190 of the subjects (6). In this group, SEBM thickness was < 10 µm in 171 cases, between 10-15 µm in 12 cases, and > 15 µm in 7 cases. Six of the 7 patients with collagen band thickness > 15 µm and 6 of the 12 patients with thickness between 10-15 µm had diarrhea. The maximum SEBM thickness recorded by Gledhill and Cole was 80 µm, and the average in the 190 patients was 22.5 µm. Consistent with our study, the authors found that SEBM thickness > 10 µm was associated with non-specific colitis, colonic diverticula, and epithelial polyp; however, they presented no data related to inflammation[6]. All the cases in our study exhibited SEBM, that is, no 0 µm values were recorded. The lowest value of SEBM thickness we observed was 3 µm, and the intensity of inflammation predominant in the tissue section with SEBM thickness of 3 µm was 0-1. Gledhill and Cole found that SEBM thickness was not correlated with age or sex[6], similar to our results.
Our aims in this study were to determine the normal limits of SEBM thickness in the southern Anatolia region of Turkey, and to investigate for possible links between SEBM thickness and age, and sex. Studies of collagenous colitis patients in Europe and the United States have revealed substantially different SEBM thickness in these geographic regions. The nutritional habits of people in south and southeast Anatolia differ from those in Europe and the United States. The main nutritional sources in southern Turkey are animal protein and wheat products. The prevalence of parasitic infection, and particularly amebiasis, is very high in this area. In one study of 35 172 patients with diarrhea who were from south and southeast Anatolia, 26.4% had amebiasis (19). The same authors investigated infectious diseases transmitted by drinking water in a study that was conducted between 1994 and 1995 in southern Turkey. The results showed 7 281 new amebiasis cases during this 1-yr period, and revealed a rise in incidence of up to 41% during the summer[20]. In our study, histological examination revealed colonic amebiasis in only 3 of 100 cases. In these 3 patients, rectal SEBM thickness ranged from 10-12 µm and the rectal biopsies all showed an eosinophil-rich inflammatory response.
Patients with collagenous colitis show different SEBM thickness in different segments of the colon. Most studies have shown that this collagenous layer is thicker in the distal colon than in the proximal regions[3,5-7,11]. Research has also revealed that patients with collagen deposition of at least 15-µm in thickness in more than 30% of multiple colon biopsies eventually develop the clinical syndrome[1,3]. In our study, the SEBM was thickest in the rectum, followed by the transverse colon, descending colon, ascending colon, and cecum, respectively. The same sequence did not apply to intensity of inflammation, i.e, cecum was the second site exhibiting relatively higher count of inflammatory cell after rectum compared to other sites. This discordance may be explained by the absence of morphometric analysis, which could give more specific result about the count of inflammatory cells than routine light microscopic evaluation. Inflammatory response, parasitic infection such as amebiasis, and nutritional factors are all elements that potentially contribute to increased basement membrane thickness. Two of our patients with SEBM thickness between 12-15 µm in any colonic segment exhibited polyp and colonic diverticula, respectively. In 2 other cases with 15-20 µm SEBM thickness, there was a history of NSAID treatment but no history of diarrhea. The colon biopsies from the latter 2 patients showed moderate inflammatory response with eosinophils predominant in the infiltrate.
In summary, of the five colon segments studied in this patient group from southern Turkey, SEBM was thickest in the rectum. Our results indicate that, in this population, SEBM thickness is not correlated with age or sex, but is positively correlated with intensity of inflammation. This study provides more evidence that SEBM thickness varies according to geographic region, and that these differences may be culture-related. The findings also suggest that measuring SEBM thickness at one segment of the colon is inadequate and may be misleading. The mean SEBM thickness for the separate colon segments differ, similar to established characteristics of collagenous colitis. The histopathological diagnosis of collagenous colitis should be made by measuring collagen band thickness in at least 3 or more non-tangential areas that are distant from the crypts. In addition to collagen band thickness, diagnosis of this condition must be based on characteristic clinical and histologic findings. The latter include superficial desquamation, intraepithelial lymphocytosis, and epithelial vacuolization.
Edited by Zhu LH and Xu FM
| 1. | Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN. Non-neoplastic colon disease. In: Gastrointestinal Pathology. 2nd ed, Lippincott-Raven, Philadelphia. 1999;153-236. |
| 2. | Bohr J, Olesen M, Tysk C, Järnerot G. Collagenous and lymphocytic colitis: a clinical and histopathological review. Can J Gastroenterol. 2000;14:943-947. [PubMed] |
| 4. | Carpenter HA, Tremaine WJ, Batts KP, Czaja AJ. Sequential histologic evaluations in collagenous colitis. Correlations with disease behavior and sampling strategy. Dig Dis Sci. 1992;37:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Bohr J. A review of collagenous colitis. Scand J Gastroenterol. 1998;33:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Gledhill A, Cole FM. Significance of basement membrane thickening in the human colon. Gut. 1984;25:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 7. | Halaby IA, Rantis PC, Vernava AM, Longo WE. Collagenous colitis: pathogenesis and management. Dis Colon Rectum. 1996;39:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Tremaine WJ. Diagnosing collagenous colitis: does it make a difference? Eur J Gastroenterol Hepatol. 1999;11:477-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Tanaka M, Mazzoleni G, Riddell RH. Distribution of collagenous colitis: utility of flexible sigmoidoscopy. Gut. 1992;33:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 114] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Cammarota G, Pignataro F, Cuoco L, Cianci R, Carbone A, Gasbarrini G, Rapaccini GL. EUS in the diagnosis of collagenous colitis. Gastrointest Endosc. 2001;54:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Offner FA, Jao RV, Lewin KJ, Havelec L, Weinstein WM. Collagenous colitis: a study of the distribution of morphological abnormalities and their histological detection. Hum Pathol. 1999;30:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Jessurun J, Yardley JH, Lee EL, Vendrell DD, Schiller LR, Fordtran JS. Microscopic and collagenous colitis: different names for the same condition? Gastroenterology. 1986;91:1583-1584. [PubMed] |
| 13. | Bogomoletz WV, Adnet JJ, Birembaut P, Feydy P, Dupont P. Collagenous colitis: an unrecognised entity. Gut. 1980;21:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | van den Oord JJ, Geboes K, Desmet VJ. Collagenous colitis: an abnormal collagen table? Two new cases and review of the literature. Am J Gastroenterol. 1982;77:377-381. [PubMed] |
| 15. | Schiller LR. Pathophysiology and treatment of microscopic-colitis syndrome. Lancet. 2000;355:1198-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Loffeld RJ, Balk AT. Subepithelial collagen thickening in the colon: report on a series of 23 patients. Digestion. 1998;59:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Lee E, Schiller LR, Vendrell D, Santa Ana CA, Fordtran JS. Subepithelial collagen table thickness in colon specimens from patients with microscopic colitis and collagenous colitis. Gastroenterology. 1992;103:1790-1796. [PubMed] |
| 18. | Jessurun J, Yardley JH, Giardiello FM, Hamilton SR, Bayless TM. Chronic colitis with thickening of the subepithelial collagen layer (collagenous colitis): histopathologic findings in 15 patients. Hum Pathol. 1987;18:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 152] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Demirhindi H, Akbaba M, Bahcebaci T, Karaomerlioglu A, Yalcin E; Situation of intestinal amebiasis among diarrheal diseases in Adana region, Cukurova, Turkey. Fifth International Conference on Travel Medicine. March 24-27, 1997. Geneva, Switzerland. . |
| 20. | Bahcebaci T, Demirhindi H, Akbaba M, Piskin A, Cegil K; Health and quality of drinking water in Adana city centre, Turkey. In-ternational Conference on Health, Environment and Development. October 14-17, 1996. Alexandria, Egypt. . |