Published online Mar 1, 2004. doi: 10.3748/wjg.v10.i5.694
Revised: August 9, 2003
Accepted: August 16, 2003
Published online: March 1, 2004
AIM: Taurine has been shown to be an effective scavenger of hypochlorous acid (HOCl). The role of HOCl is well established in tissue damage associated with inflammation and injury. In the present study, the effect of HOCl on nuclear nucleoside triphosphatase of hepatocytes and the ability of taurine to prevent this effect were investigated.
METHODS: Isolated hepatic nuclei from rat liver were exposed to HOCl with or without taurine. The NTPase activity on nuclear envelope was assayed using ATP and GTP as substrates, respectively.
RESULTS: The first series of experiments evaluated the toxicity of HOCl and the efficacy of taurine to protect NTPase. HOCl at 10-9-5 × 10-6 mol/L reduced nuclear NTPase activities in a concentration dependent manner (ATP and GTP as substrates) (P < 0.01). HOCl at 10-6 mol/L reduced the NTPase activity by 65% (ATP as substrate) and 76% (GTP as substrate). Taurine (10-7 to 10-4 mol/L) was tested for protection against HOCl at 10-6 mol/L and the nuclei treated with 5 × 10- 4 mol/L taurine exhibited only 20% and 12% reduction in NTPase activities compared to untreated controls. A second study was performed comparing taurine to glutathione (GSH). GSH and HOCl at 10-6 mol/L exhibited 46% and 67.4% reduction in NTPase activities compared with control. GSH (10-4 mol/L) which was incubated with the nuclei and HOCl still exhibited 44.2% and 44.8% reduction in NTPase activities of untreated control. Taurine with HOCl only exhibited 15.2% and 17.1% reduction in NTPase activities, which provided more powerful protection against HOCl than GSH. The third experiment was undertaken to evaluate the specificity of taurine against HOCl. Incubation of rat hepatic nuclei with Fe3+/H2O2 (1 mmol/L vs 5 µmol/L) resulted in a decrease in nuclear NTPase activities (P < 0.01). When hepatic nuclei were incubated with Tau (10-4 mol/L) and Fe3+/H2O2 (1mmol/L vs 5 µmol/L), nuclear NTPase activities were only slightly increased as compared with that of incubation with Fe3+/H2O2 alone. However, GSH failed to alter the NTPase activities induced by Fe3+/H2O2.
CONCLUSION: The present findings indicate that HOCl can act as an inhibitor of nuclear NTPase. Taurine can antagonistically reduce the toxicity of HOCl to NTPase.
- Citation: Li JX, Pang YZ, Tang CS, Li ZQ. Protective effect of taurine on hypochlorous acid toxicity to nuclear nucleoside triphosphatase in isolated nuclei from rat liver. World J Gastroenterol 2004; 10(5): 694-698
- URL: https://www.wjgnet.com/1007-9327/full/v10/i5/694.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i5.694
The mechanism of mRNA transport involves two major steps: the recognition of RNA molecules to be transported and their transfer through the nuclear pore. The latter step is an important rate-limiting step in protein expression[1]. The nucleocytoplasmic transport of mRNA is an energy-consuming process. The energy requirement is associated with the functioning of a nucleoside triphosphatase (NTPase). The nuclear NTPase activity exhibits a broad substrate specificity toward nucleotides and divalent metal cations[2,3]. The recent data demonstrated that the activity of the NTPase was strikingly inhibited by cholesterol oxidase treatment, which indicated that oxidation of nuclear membrane cholesterol could inhibit NTPase activity[4]. These results have implications for mRNA flux across the nuclear membrane during conditions where lipid peroxidation may be expected.
Hypochlorous acid (HOCl) is a major oxidant produced by neutrophils and monocytes, via the myeloperoxidase-catalyzed oxidation of chloride by hydrogen peroxide[5]. HOCl is a potent oxidant capable of damaging host tissue during inflammation. The strong oxidizing species HOCl plays a highly significant role in the bactericidal function of the neutrophil. However, inappropriate and/or excessive activation of neutrophils leads to oxidative stress and collateral damage to surrounding tissues. Cysteine and methionine residues in proteins and reduced glutathione (GSH) appear to be the main targets for HOCl[6], thereby altering the structure and function of proteins and lowering antioxidant status in the cell. In the literature, taurine, a 2-amino ethanesulfonic acid, is characterized as an antioxidant, a membrane protector, or a regulator of calcium ion homeostasis. It is the major free intracellular amino acid that presents in many tissues[7,8] and possibly acts physiologically as a trap for HOCl[8]. In the present study, we explored the possible action of HOCl on hepatic nuclear NTPase activity and the protective effect of taurine on the changes of NTPase activity induced by HOCl.
Male Sprague-Dawley (SD) rats were supplied by the Animal Center, Health-Science Center, Peking University. Taurine and GSH were purchased from Sigma Chemical Co (St. Louis, MO, USA). The term HOCl was used to cover the equilibrium mixture with OCI- present at neutral pH. The following reagents were freshly prepared. Phenylmethylsulfonyl fluoride (PMSF), sodium salt of nucleotides (ATP and GTP); DS/PMSF buffer (mmol/L): 250 sucrose, 50 Tris/HCl pH7.4, 5 MgCl2, 1 PMSF; STM/ Buffer (mmol/L): 2100 sucrose, 50 Tris/HCl pH7.4, 5 MgCl2, 1 PMSF, 1 EDTA, l DTT, and l µmol/L leupeptin. All the reagents were analytically pure.
Rat hepatocytes were isolated according to Berry and Friend methods[9]. Briefly, under anesthesia with urethane (1 g/kg i.p.), male SD rats (220-250 g) were in situ liver-perfused at 37 °C via portal vein, with Ca2+-free Hanks’ solution containing 5 mg/L collagenase and 1 mg·L-1 hyaluronidase bubbling of 950 ml/L O2-50 ml/L CO2. After 20 min perfusion, the liver was removed, transferred to a beaker containing 200 mL of enzyme medium, broken up with a blunt spatula, and shaken at 37 °C for 15 min in an atmosphere of air. The suspension was filtered through nylon mesh and the cells were separated from debris by centrifuging at 50 g for 2 min. The cells were resuspended in Hanks’ solution at 4 °C. Cell viability tested by trypan blue exclusion was higher than 90%.
Isolation of rat liver nuclei was performed according to the method described by Kaufmann et al[10] with modification. Suspended cells were homogenized in Teflon (10 strokes), sedimented at 800 r/min for 10 min. The nuclei were suspended in DS/PMSF buffer, layered over cushions of this buffer, and sedimented at 70000 g for 60 min. Isolated nuclei were resuspended in STM/PMSF buffer, again layered over cushions of DS/PMSF buffer, and sedimented at 70000 g for 30 min. The final pellet was resuspensed with STM/PMSF to 1 mg protein/mL, and stored at -70 °C.
Nuclear membrane NADH pyrophosphorylase activity and microsome-NADPH cytochrome-C reductase activity were determined to test the purification of the freshly isolated hepatocyte nuclei.
Isolated purified nuclei (0.25 mL) were incubated with different chemicals (dissolved in 0.25 mL) for 10 min at 30 °C. The reaction was stopped by cold (4 °C) centrifugation on microcentrifuge for 2 min, and the nuclei pellet was washed once and then resuspended in STM/PMSF to obtain a final protein concentration of 1 mg/mL.
Protocol 1: incubation with buffer alone (control) and sodium hypochlorite (10-9 to 5 × 10-6 mol/L), respectively. Protocol 2: Incubation with buffer alone (control), taurine (10-6, 10-5 and 10-4 mol/L), sodium hypochlorite (10-6 mol/L), sodium hypochlorite (10-6 mol/L) plus taurine (10- 7 to 10-4 mol/L), respectively. Protocol 3: Incubation with sodium hypochlorite (10-6 mol/L), sodium hypochlorite (10-6 mol/L) plus glutathione (GSH, 10-6 to 10-4 mol/L), respectively. Protocol 4: Incubation with buffer alone (control), taurine (10-4 mol/L), GSH (10-4 mol/L), H2O2/FeSO4 (1mmol/L/5 µmol/L), H2O2/FeSO4 (1 mmol/L/5 µmol/L) plus taurine (10-6 to 10-4 mol/L), H2O2/FeSO4 (1 mmol/L/5 µmol/L) plus GSH (10-6 to 10-4 mol/L), respectively.
NTPase activity was assayed as described by Tiffany[11] and Ramjiawan[12] with modification. Nuclear suspension (1 µg protein/µL) was preincubated for 10 min at 30 °C. Addition of 1.0 mmol/L ATP or 1.0 mmol/L GTP initiated the reaction. Ten minutes after 30 °C-incubation, the reaction was stopped by addition of 100 g/L SDS and placing the test tube on ice bath, and inorganic phosphate was measured according to the method of Raess[13], which was expressed as nmol/mgPr per 10 min. Preliminary experiments showed a linear relationship of NTPase activity with incubation time of nucleoside triphosphate within 30 min. The values were normalized to protein content.
Separated six experiments were performed in duplicate. All results were expressed as mean ± SD. Statistical analysis of the data was performed using one-way analysis of variance followed by Student-Newman-Keuls tests. P < 0.05 was accepted as statistically significant.
The level of NADH pyrophosphorylase activity (as marker enzyme for nuclear envelope) in prepared nuclei from rat hepatocytes was 7-fold that in homogenate of whole cells (25.77 ± 1.26 vs 3.68 ± 0.27 nmol/mg Pr per min, P < 0.01), but NADPH cytochrome C reductase activity (marker enzyme for microsome) was only 28% of that in hypatocytes homogenate (2.88 ± 0.22 vs 10.27 ± 0.87 nmol/mg Pr per min, P < 0.01). While the activity of mannose-6-phosphatase existing in both microsomes and nuclei, was 4-5 times that in cell homogenate (412 ± 22 vs 91 ± 6 nmol/mg Pr per min, P < 0.01). It showed that the isolated hepatic nuclear fraction was of high purity and little contaminated by other organelles.
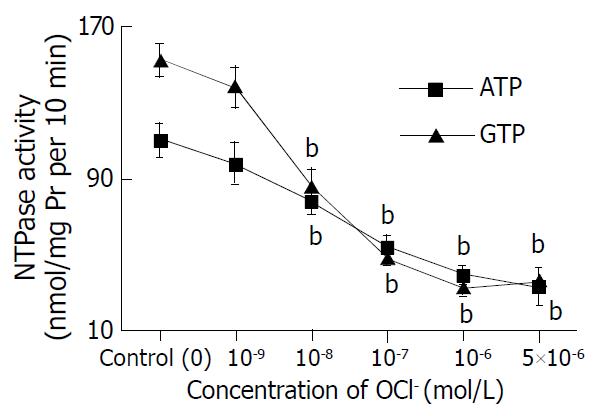
HOCl (at mol/L: 10-9-5 × 10-6) could significantly depress NTPase activity of hepatic nuclei in a concentration- dependent manner, regardless ATP or GTP as a substrate (Figure 1). After incubation of hepatic nuclei with 5 × 10-6 mol·L-1 HOCl, the hepatic nuclear NTPase activities were decreased by 70.0% (ATP as substrate) and by 76.3% (GTP as substrate), compared with those of control groups (both P values less than 0.01) respectively.
The effect of taurine on NTPase activity is shown in Table 1. After incubation of hepatic nuclei with different concentrations of taurine (10-6, 10-5 and 10-4 mol/L), the NTPase activities on nuclear envelope were increased in a concentration-dependant fashion, either using ATP or GTP as a substrate (all P values < 0.05 as compared with those of controls). When taurine was at 10-4 mol/L, the NTPase activities were increased by 18.1% (ATP as substrate) and 27.3% (GTP as substrate), respectively. All P values were less than 0.01 as compared with those of their controls.
The abilities of HOCl to depress NTPase were confirmed by detecting NTPase activities. Incubation of hepatic nuclei with HOCl at 10-6 mol·L -1 resulted in an obviously lower nuclear NTPase activity than that with buffer alone. The hepatic nuclear NTPase activities were decreased by 65.4% (ATP as substrate) and by 76.0%(GTP as substrate), compared with the control groups respectively (P < 0.01).
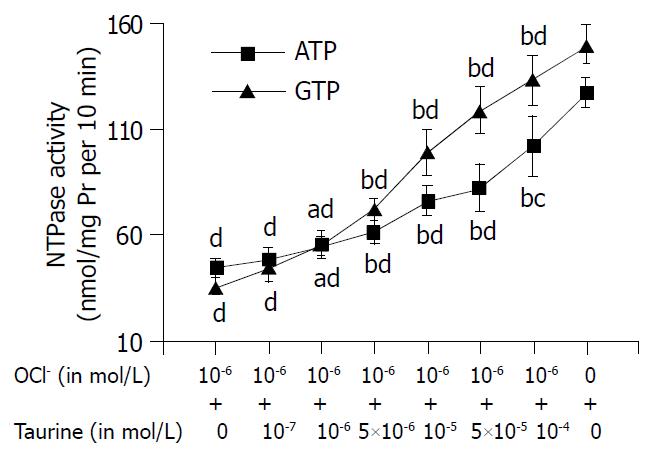
The reduction of NTPase activities induced by HOCl was antagonized by taurine (as shown in Figure 2), even at a very low concentration (10-6 mol/L) (ATP and GTP as substrates). The antagonistic effect of taurine on HOCl was in a concentration dependent manner. When the nuclei were incubated with HOCl (10-6 mol/L) and taurine (5 × 10-4 mol/L), the NTPase activity reached 80.3% (ATP as substrate) and 88.7% of control group (GTP as substrate), respectively (all P values less than 0.01).
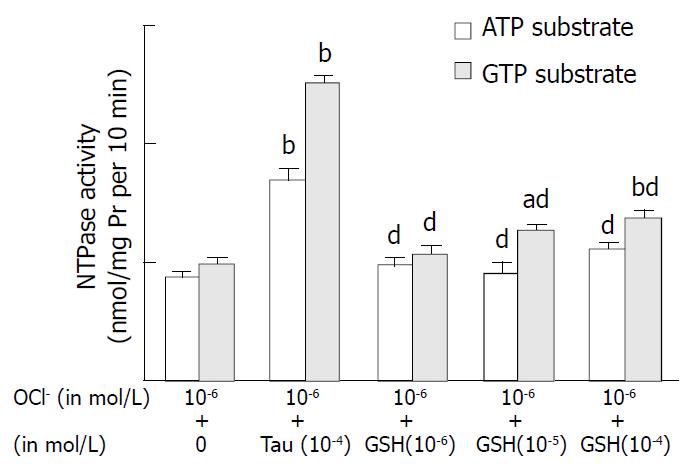
Incubation of hepatic nuclei with HOCl at 10-6 mol/L resulted in an obviously lower nuclear NTPase activity. The hepatic nuclear NTPase activities were decreased by 51.2% (ATP as substrate) and by 101.3% (GTP as substrate), compared with the control groups respectively (P < 0.01). The reduction of NTPase activities induced by HOCl was antagonized by taurine (10-4 mol/L, ATP and GTP as substrates). Incubation of taurine increased the NTPase activity by 92.6% (ATP as substrate) and 154% (GTP as substrate) compared with HOCl incubation (as shown in Figure 3). GSH incubation attenuated the depressive effect of HOCl in a concentration- dependent manner. When the nuclei were incubated with HOCl (10-4 mol/L) and GSH (10-4 mol/L), the NTPase activity was increased by 27% (ATP as substrate) and 38.5% (GTP as substrate) of HOCl incubation group, respectively (all P values less than 0.01). It was showed that the effect of GSH on HOCl-induced depression of NTPase was smaller than that of taurine (F value: 5.3, P < 0.01).
The ability of the Fe3+-H2O2 system to produce ·OH was confirmed by detecting NTPase activities. Incubation of hepatic nuclei with Fe3+-H2O2 at 1 mmol/L/5 µmol/L resulted in a lower nuclear NTPase activity as compared with buffer alone. The NTPase activities on nuclear envelopes were decreased by 70% (ATP as substrate) and by 76.7% (GTP as substrate), compared with control group respectively (Table 2).
| Groups | NTPase activity (nmol/mg Pr per 10 min) | |
| ATP as substrate | GTP as substrate | |
| Control | 100.0 ± 9.9 | 151.8 ± 9.9 |
| Tau (10-4 mol/L) | 138 ± 14.6 | 175.5 ± 5.9 |
| GSH (10-4 mol/L) | 95 ± 12.2 | 150.0 ± 9.8 |
| OH | 29.8 ± 8.2b | 35.3 ± 7.8b |
| OH+Tau (10-6 mol/L) | 35.6 ± 6.1 | 36.2 ± 8.8 |
| OH+Tau (10-5 mol/L) | 40.8 ± 8.8 | 32.7 ± 7.3 |
| OH+Tau (10-4 mol/L) | 46.5 ± 8.7a | 43.3 ± 7.2 |
| OH+GSH (10-6 mol/L) | 32.0 ± 8.2 | 43.2 ± 11.8 |
| OH+GSH (10-5 mol/L) | 32.1 ± 9.7 | 41.2 ± 9.6 |
| OH+GSH (10-4 mol/L) | 44.3 ± 6.2 | 39.8 ± 5.9 |
The reduction of NTPase activities induced by Fe3+-H2O2 was antagonized by taurine. When taurine was at 10-4 mol/L (ATP as substrate), the decreases of NTPase activities induced by Fe3+-H2O2 (1 mmol/L/5 µmol/L) were slightly reversed (from 29.8 ± 8.2 to 46.5 ± 8.7, P < 0.05). Whereas, GSH, at all concentrations used in our experiment, had no significant effect on Fe3+-H2O2 -induced depression of NTPase activity (Table 2).
Nuclear NTPase, a nuclear membrane-associated enzyme, provides energy for poly (A)+mRNA export through the nuclear pore. Many factors may play a modulatory role in NTPase activity. Extracellular biological active molecules, such as insulin, epidermal growth factor and nuclear membrane cholesterol, could affect NTPase activities through the individual cellular signal transduction system[14]. In addition, oxygen derived free radicals of nuclear membrane cholesterol could inhibit nucleoside triphosphatase activity[4]. Thus, export of poly (A) mRNA from the nucleus via the nuclear pore complex was influenced, which plays a crucial role in protein synthesis[1-3,14].
In this present study using nuclei purified from rat hepatocytes, HOCl was confirmed to be a very efficient inhibitor of nuclear NTPase activity. Hepatic nuclear NTPase activity was depressed by incubation of hepatic nuclei with HOCl in a concentration dependent manner, regardless of using ATP or GTP as substrate. It was suggested that NTPase was one of the favorite targets of HOCl. The inhibition of this enzyme might probably be caused by oxidation of an amino acid critical for enzyme function. It is difficult to determine the exact concentration of HOCl that can be reached in vivo since it is formed locally and HOCl is very reactive. Concentrations of the drugs in the present study were not quite inadequately used. In our experiments, taurine and GSH were present which might repair the oxidative damage to the NTPase. Therefore the inhibition of nuclear NTPase activity in vitro was reversible. Furthermore, taurine has been found to be an activator for nuclear NTPase, since it could stimulate hepatic nuclear NTPase activity in a concentration dependent manner. Taurine and thiol group-containing compounds could play a protecting role during inflammatory processes.
The mechanisms of the effect of HOCl were not concerned in the present studies. It has been shown that HOCl is highly reactive with a wide range of biological molecules[15,16]. Of these, thiols are among the most reactive and crucial targets for oxidation in a cell. The deleterious effects of HOCl could be prevented by incubating the nuclei with thiol group-containing compounds as glutathione in the present study. This was in perfect agreement with Pullar et al[6] who reported that HOCl could react rapidly with thiol groups. The initial product of oxidation of thilos by HOCl was sulfinyl chloride[17]. It could react with additional thiols to give disulfide[17]. Oxidation of sulfhydryl groups in proteins might affect their functional properties. Formation of protein disulfides, mixed disulfides with GSH, or sulfinic acids could result in changes in enzymatic activity, conformation or affinity toward other molecules. Such changes could contribute to the cell damage caused by oxidative stress[18].
As an antioxidant, taurine could effectively antagonize the toxic effect of HOCl on NTPase. However, the mechanism of this effect remains unclear. More recent information has revealed that taurine could interact with peroxide anions to form stable products TauCl[8]. The latter was the product formed through the sequestration of taurine with HOCl and has been found to be an exceptionally stable and long-lived compound with cytoprotective properties due to its ability to preserve cellular function in response to physiologic stress[7]. In the present study, taurine greatly inhibited the suppression of hepatic nuclear NTPase activity induced by OCl-, indicating the important protective role of taurine against OCl- attack.
It has been found that oxygen free radical species such as H2O2 and O2 are produced in mammalian cells during normal aerobic metabolism[19,20]. However, O2 or H2O2 dose not directly act under physiologically relevant conditions. It has been proposed that much of the toxicity of these species in living organisms be due to the iron-dependent generation of ·OH, and /or other powerful oxidants, by Fenton chemistry[21]. Once it oxidizes Fe2+, the reactive ·OH is produced. Incubation of hepatic nuclei with Fe3+-H2O2 in the present study resulted in the decrease of NTPase activities in a concentration dependent manner both using ATP and GTP as substrates, which was coincident with that of Ramjiawan’s work[4]. The results of this in vitro study demonstrated that neither taurine nor GSH could directly prevent the reduction of nuclear NTPase activity caused by the ·OH producing Fe3+/H2O2 system, even if very high concentrations of them (10-4 mol·L-1) were used regardless of using ATP or GTP as substrate. These results therefore suggested that taurine could protect NTPase from HOCl specifically.
It has been found that HOCl is produced under aerobic and pathophysiological conditions such as oxidative stress and inflammation[22]. Under most circumstances, HOCl is likely to be the major strong oxidant produced by neutrophils, and contributors to oxidative damages associated with a variety of diseases in which inflammatory cells participate[23]. Impairment of NTPase on hepatic nuclei by HOCl might result in default of RNA nucleocytoplasmic transport. Taurine could antagonize the toxic effect of HOCl on NTPase. This observation could be a part of the global machinery, which acts as a cytoprotective factor in liver inflammation and oxygen stress.
In summary, our results showed that HOCl could cause a decrease in nuclear NTPase activities, which was most likely the result of decreased breakdown of NTPase. This pointed toward HOCl as an inhibitor of this enzyme. Nuclear NTPase can be effectively protected by taurine against HOCl driven oxidative injury, a consequence of direct drug scavenging capacity towards HOCl. Interaction of taurine with HOCl can also protect nuclear NTPase activity. Therefore, taurine treatment would have a beneficial effect on some diseases relating to protein synthesis.
Edited by Wang XL Proofread by Zhu LH
| 1. | Izaurralde E, Mattaj IW. RNA export. Cell. 1995;81:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 169] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Tomassoni ML, Amori D, Magni MV. Changes of nuclear membrane lipid composition affect RNA nucleocytoplasmic transport. Biochem Biophys Res Commun. 1999;258:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Agutter PS. Influence of nucleotides, cations and nucleoside triphosphatase inhibitors on the release of ribonucleic acid from isolated rat liver nuclei. Biochem J. 1980;188:91-97. [PubMed] |
| 4. | Ramjiawan B, Czubryt MP, Massaeli H, Gilchrist JS, Pierce GN. Oxidation of nuclear membrane cholesterol inhibits nucleoside triphosphatase activity. Free Radic Biol Med. 1997;23:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 241] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Pullar JM, Winterbourn CC, Vissers MC. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am J Physiol. 1999;277:H1505-H1512. [PubMed] |
| 7. | Lourenço R, Camilo ME. Taurine: a conditionally essential amino acid in humans An overview in health and disease. Nutr Hosp. 2002;17:262-270. [PubMed] |
| 8. | Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101-163. [PubMed] |
| 9. | Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3387] [Cited by in RCA: 3600] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 10. | Kaufmann SH, Gibson W, Shaper JH. Characterization of the major polypeptides of the rat liver nuclear envelope. J Biol Chem. 1983;258:2710-2719. [PubMed] |
| 11. | Tiffany BR, White BC, Krause GS. Nuclear-envelope nucleoside triphosphatase kinetics and mRNA transport following brain ischemia and reperfusion. Ann Emerg Med. 1995;25:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ramjiawan B, Czubryt MP, Gilchrist JS, Pierce GN. Nuclear membrane cholesterol can modulate nuclear nucleoside triphosphatase activity. J Cell Biochem. 1996;63:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Raess BU, Vincenzi FF. A semi-automated method for the determination of multiple membrane ATPase activities. J Pharmacol Methods. 1980;4:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Schröder HC, Wenger R, Ugarković D, Friese K, Bachmann M, Müller WE. Differential effect of insulin and epidermal growth factor on the mRNA translocation system and transport of specific poly(A+) mRNA and poly(A-) mRNA in isolated nuclei. Biochemistry. 1990;29:2368-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 250] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Prütz WA, Kissner R, Koppenol WH, Rüegger H. On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids. Arch Biochem Biophys. 2000;380:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic Biol Med. 2001;30:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709-720. [PubMed] |
| 19. | Jaeschke H. Mechanisms of oxidant stress-induced acute tissue injury. Proc Soc Exp Biol Med. 1995;209:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Simpkins CO. Metallothionein in human disease. Cell Mol Biol (Noisy-le-grand). 2000;46:465-488. [PubMed] |
| 21. | Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3121] [Cited by in RCA: 2964] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 22. | Bomzon A, Ljubuncic P. Oxidative stress and vascular smooth muscle cell function in liver disease. Pharmacol Ther. 2001;89:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 290] [Article Influence: 11.6] [Reference Citation Analysis (0)] |