Published online Feb 15, 2004. doi: 10.3748/wjg.v10.i4.560
Revised: November 18, 2003
Accepted: December 16, 2003
Published online: February 15, 2004
AIM: To investigate the effects of Ginkgo biloba extract on cytoprotective factors in rats with duodenal ulcer.
METHODS: Sprague-Dawley rats were randomly divided into four groups: sham operation without ginkgo, sham operation with ginkgo, duodenal ulcer without ginkgo, and duodenal ulcer with ginkgo. Rats with duodenal ulcer were induced by 500 mL/L acetic acid. Rats with ginkgo were intravenously injected with Ginkgo biloba extract from the tail at a dose of 0.5 mg/(kg·d) for 7 and 14 days.
RESULTS: Pathological result showed that duodenal ulcer rats with ginkgo improved mucosal healing and inflammation compared with those without ginkgo after 7 d treatment. After 14 d treatment, duodenal ulcer rats with ginkgo significantly increased weight gain (34.0 ± 4.5 g versus 24.5 ± 9.5 g, P < 0.05) compared with those without ginkgo. Duodenal ulcer rats significantly increased cell proliferation (27.4 ± 4.0 and 27.8 ± 2.3 BrdU-labeled cells in duodenal ulcer rats with and without ginkgo versus 22.4 ± 3.5 and 20.8 ± 0.5 BrdU-labeled cells in sham operation rats with and without ginkgo, P < 0.05) compared with sham operation rats. Mucosal prostaglandin E2 concentration significantly increased by 129% (P < 0.05) in duodenal ulcer rats with ginkgo compared with that in those without ginkgo. Duodenal ulcer rats without ginkgo significantly decreased superoxide dismutase activity in the duodenal mucosa and erythrocytes (19.4 ± 6.7 U/mg protein versus 38.1 ± 18.9 U/mg protein in the duodenal mucosa, and 4.87 ± 1.49 U/mg protein versus 7.78 ± 2.16 U/mg protein in erythrocytes, P < 0.05) compared with sham operation rats without ginkgo. However, duodenal ulcer rats with ginkgo significantly increased erythrocyte superoxide dismutase activity (8.22 ± 1.92 U/mg protein versus 4.87 ± 1.49 U/mg protein, P < 0.05) compared with those without ginkgo. Duodenal ulcer rats without ginkgo significantly increased plasma lipid peroxides (4.18 ± 1.12 μmol/mL versus 1.60 ± 1.10 μmol/mL and 1.80 ± 0.73 μmol/mL, P < 0.05) compared with sham operation rats without ginkgo and duodenal ulcer rats with ginkgo during the experimental period.
CONCLUSION: Ginkgo biloba extract can improve weight gain and mucosal healing in duodenal ulcer rats by the actions of cytoprotection and antioxidation.
- Citation: Chao JCJ, Hung HC, Chen SH, Fang CL. Effects of Ginkgo biloba extract on cytoprotective factors in rats with duodenal ulcer. World J Gastroenterol 2004; 10(4): 560-566
- URL: https://www.wjgnet.com/1007-9327/full/v10/i4/560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i4.560
Extract from the leaves of Ginkgo biloba (maidenhair tree) has been used therapeutically for centuries in the traditional Chinese pharmacopeia, and is used for the treatment of asthma and bronchitis[1]. In Western countries, Ginkgo biloba extract is administrated as film-coated tablets, a liquid, or by intravenous injection. In Germany and France, Ginkgo biloba extract is the most commonly prescribed medicine to improve circulation and cardiovascular health[1]. Standard Ginkgo biloba extract, EGb 761, contains 240 mg/g flavonoids (ginkgo-flavone glycosides) and 60 mg/g terpenoids (ginkgolides and bilobalides) which are the most important active ingredients in the extract. Ginkgo biloba extract is well known for its antioxidant property to scavenge free radicals, especially oxygen-centered free radicals, such as OH·, O2·-, RO·, and ROO·, and to neutralize ferryl ion-induced peroxidation[2]. It has been reported that the antioxidant activity of Ginkgo biloba extract can prove helpful in the prevention and treatment of diseases and degenerative processes associated with oxidative stress.
Oxygen-derived radicals are involved in the pathogenesis of duodenal ulceration[3]. A previous study found that the pathogenesis of duodenal ulcer induced by diethyldithiocarbamate in rats could be significantly prevented by subcutaneous injection of antioxidative agents, such as superoxide dismutase (SOD, 50000 units/kg), allopurinol (50 mg/kg), or glutathione (200 mg/kg), and antisecretory agent cimetidine (100 mg/kg)[4]. Additionally, SOD injection significantly elevated the rate of alkaline secretion in the duodenum, suggesting that the mucosal antioxidative system including SOD may play a role in the regulation of alkaline secretion and contribute to duodenal mucosal defensive ability. In cysteamine-induced duodenal ulcer rats, Cu, Zn-SOD activity significantly decreased, but lipid peroxidation products determined by thiobarbituric acid reactants significantly increased[5]. The results suggest that an increase of oxygen-derived free radicals and a decrease of Cu, Zn-SOD activity in the duodenal mucosa may be involved in the pathogenesis of cysteamine-induced duodenal ulcer. Non-smokers with peptic ulcer, clinically diagnosed as gastric or duodenal ulcer, had a higher level of malondialdehyde (MDA) in platelets, whereas platelet SOD activity significantly decreased compared with healthy subjects[6].
Several mechanisms of gastroduodenal cytoprotection have been proposed, including increased formation of prostaglandins and scavenging of free radicals, to protect against gastroduodenal mucosal injury rather than inhibition or neutralization of gastric acid[7]. There is no study yet to investigate the effect of Ginkgo biloba extract on peptic ulcer. Presumably, the property of a free radical scavenger for Ginkgo biloba extract can protect duodenal ulcer from oxidative damage. Therefore, the purpose of this study was to investigate the effect of Ginkgo biloba extract on cytoprotective factors in rats with acetic acid-induced duodenal ulcer.
Male Sprague-Dawley rats (200-250 g) were purchased from the National Laboratory Animal Center (National Science Council, Taipei, Taiwan). The rats were housed in individual cages and had free access to food (powdered laboratory autoclavable rodent diet 5010, PMI Nutrition International Inc., Brentwood, MO), except for the fasting period. The light cycle was 12 hours and the room temperature was kept at 22-24 °C. One hundred and forty-four rats were randomly divided into four groups: sham operation without ginkgo, sham operation with ginkgo, duodenal ulcer without ginkgo, and duodenal ulcer with ginkgo (n = 36 per group). Rats with duodenal ulcer were induced by acetic acid[8]. Prior to operation, the rats were fasted overnight, anesthetized by intraperitoneal injection with 50 mg/kg body weight thiopental sodium (Abbott Australasia Pty.Ltd., Kurnell, Australia), and the abdomen was then opened. A plastic tube (4.5 mm in inner diameter) filled with 70 μL of 500 mL/L acetic acid was applied tightly to the surface of the duodenum for 15 s. Due to different tolerance to acetic acid in various layers of the duodenum, it only caused immediate necrosis in the mucosa and submucosa, and a part of muscular layers exactly within the area of acetic acid application without penetration or perforation to the surrounding organs. Normal saline was used in sham operation rats instead of acetic acid. After operation, the rats were allowed to recover from anesthesia. Operated rats received only water on the day of operation (day 0), and were fed with a normal chow diet ad libitum next day (day 1) before treatment. Body weight of the rats was routinely recorded. All protocols were conducted under the guidelines of Animal Care and Use Committee, Taipei Medical University.
The next day after operation, the rats were intravenously (iv) injected with Ginkgo biloba extract solution (Cerenin®, Dr. Willmar Schwabe GMBH & Co., Karlsruhe, Germany) from the tail at a single dose of 0.5 mg/(kg·d) for 7 and 14 days. The dose of Ginkgo biloba extract was calculated from the clinical recommendation of 35 mg intravenous injection (5 mL injection solution) for 70 kg adults (Cerenin®). Each mL of the injection solution contained 3.5 mg Ginkgo biloba extract (240 mg/g flavonoids and 60 mg/g terpenoids), 30 mg of 960 mL/L ethanol, 40 mg sorbitol, and 0.1 mol/L NaOH. The injection volume was adjusted daily by body weight. Rats without ginkgo were intravenously injected with the same volume of vehicle solution without ginkgo from the tail. Four rats in each group were killed on day 1 before ginkgo treatment to identify the formation of duodenal ulcer, and sixteen rats in each group were sacrificed for both biochemical analyses (8 rats) and pathological examination (8 rats) after 7 and 14 d treatment, respectively. The duodenum (5 mm × 5 mm) was excised, preserved in 40 mg/L paraformaldehyde, and stained with haematoxylin and eosin. Under light microscope at a magnification of × 100 or × 200, coded specimens were evaluated by a pathologist in a blinded fashion.
Rats were intraperitoneally (ip) injected with bromodeoxyuridine (BrdU; Sigma-Aldrich Co., St. Louis, MO) 5 mg/kg body weight one hour before sacrifice for immunohistochemical analysis[9]. The duodenum (4 μm thick) was fixed in formalin and embedded in paraffin. The duodenum sections were deparaffinized in xylene, dehydrated by graded ethanol series, and placed in 0.01 mol/L phosphate buffered saline (pH 7.4). Antigen retrieval was performed by boiling the duodenum sections in 0.1 mol/L citrate buffer (pH 6.0) for 10 min to deactivate endogenous alkaline phosphatase activity. The tissue sections were then exposed to 3% H2O2 to block endogenous peroxidases, and followed by partial denaturation of double-stranded DNA by treatment with 1 mol/L HCl. The sections were incubated with monoclonal mouse anti-BrdU antibody (1:50 dilution; DAKO, Denmark) for 1 h, and incubated with biotinylated anti-mouse IgG for 30 min. The sections were stained by a streptavidin-biotin complex method using a commercial kit (LSAB2 kit/HRP, DAKO), and detected by diaminobenzidine. The number of cells in the proliferation zone was calculated by counting the cells between the first stained cells of the serosal side and the last stained cells of the mucosal surface of a crypt under light microscope to evaluate the healing of the mucosa[9]. The number of BrdU-labeled cells in the proliferation zone was counted by the number of positive cells from the serosal side to the mucosal surface of a crypt to evaluate DNA synthesis.
Prostaglandin E2 (PGE2) concentration in the duodenal mucosa was quantitated by ELISA using a commercial kit (DE2100, Research and Diagnostics Systems, Inc., Minneapolis, MN). The duodenal mucosa (100 mg wet weight) was homogenized with 1 mL of 150 mL/L methanol, and centrifuged at 8000 × g at 4 °C for 10 min. The supernatant was applied to a C18 Sep-Pak® minicolumn (Amersham Biosciences Ltd. Taiwan Branch, Taipei, Taiwan) at the flow rate of 0.5 mL/min. The column was washed with 2 mL petroleum ether followed by 2 mL of 150 mL/L methanol, and prostaglandin was eluted by 2 mL methyl formate. The elute was evaporated using nitrogen gas, and the residue was dissolved in 0.5 mL deionized water. The sample suspension or standards (100 μL) were added to a 96-well plate coated with goat anti-mouse polyclonal antibody, incubated with 50 μL prostaglandin E2 conjugated to alkaline phosphatase and 50 μL mouse monoclonal anti-prostaglandin E2 antibody at 2-8 °C for 18-24 h. After several washes, the samples were incubated with 200 μL p-nitrophenyl phosphate substrate at 37 °C for 1 h, and the reaction was terminated by 50 μL trisodium phosphate solution. The levels of prostaglandin E2 were determined at 405 nm using an ELISA reader (Multiskan RC, Thermo Labsystems, Helsinki, Finland). Protein content in the duodenal mucosa was determined by the modified method of Lowry et al[10] using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA).
The activity of SOD was determined spectrophotometrically at 525 nm using a commercial kit (Bioxytech® SOD-525TM assay, Oxis International, Inc., Portland, OR). Duodenal mucosa (15-20 mg) was homogenized in 0.25 mol/L sucrose solution, and centrifuged at 8500 × g at 4 °C for 10 min. Blood samples (200 μL) were centrifuged at 2500 × g at 4 °C for 5 min. The erythrocyte pellet was suspended in ice-cold water. The mucosa supernatant and erythrocyte suspension were extracted with ethanol/chloroform (62.6:37.5, v/v) for removal of hemoglobin interference. The samples were incubated with the chromogenic reagent (5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzo[c]fluorene) in alkaline condition (pH 8.8), and the absorbance was read at 525 nm within 1 min. The specific activity of SOD was expressed as unit/mg protein.
One SOD unit was defined as the activity that doubled the autooxidation rate in the absence of SOD.
Because of the limited amount of the duodenum specimen, lipid peroxidation was measured in the plasma. The concentration of lipid peroxides in the plasma was assessed colorimetrically at 586 nm using a commercial kit (Calbiochem 437634, Calbiochem-Novabiochem Cor., La Jolla, CA). The plasma (200 μL) was mixed with 650 μL of Reagent 1 (7.7 mM N-methyl-2-phenylindole in 750 mL/L acetonitrile and 250 mL/L methanol) and 150 μL of Reagent 2 (15.4 M methanesulfonic acid) at 45 °C for 40 min. The levels of MDA and 4-hydroxy-2(E)-nonenal (4-HNE), the end products derived from peroxidation of polyunsaturated fatty acids and related esters, were measured at 586 nm.
The data are expressed as mean ± SD. The data were analyzed by two-way ANOVA to determine the main effects of duodenal ulcer and ginkgo using SAS (version 6.12, SAS Institute, Cary, NC). Post hoc multiple comparisons between two groups were performed by Fisher’s least significant difference test. Statistical significance was assigned at the 0.05 level.
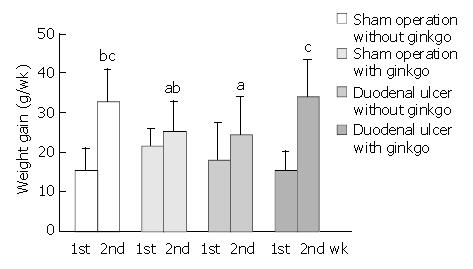
The total dose of ginkgo was 108.7 ± 4.1 mg/d, 105.3 ± 8.0 mg/d for 7 d treatment, and 106.2 ± 4.7 mg/d, 114.8 ± 3.5 mg/d for 14 d treatment in sham operation and duodenal ulcer rats, respectively. Duodenal ulcer rats for 14 d treatment had higher ginkgo administration (P < 0.05) compared with other rats. However, the total dose of ginkgo did not significantly differ among other rats. The initial weight on day 1 was 217.3 ± 19.6 g, 206.6 ± 9.1 g, 214.7 ± 15.9 g, and 202.7 ± 16.9 g for 7 d treatment, and 221.8 ± 11.4 g, 199.8 ± 10.5 g, 200.2 ± 18.6 g, and 212.6 ± 7.5 g for 14 d treatment in sham operation rats without ginkgo, sham operation rats with ginkgo, duodenal ulcer rats without ginkgo, and duodenal ulcer rats with ginkgo, respectively. The initial weight did not significantly differ among the groups. Weight gain did not significantly differ among four groups during the first week (Figure 1). Duodenal ulcer rats without ginkgo (24.5 ± 9.5 g) had significantly lower weight gain (P < 0.05) compared with sham operation rats without ginkgo (32.5 ± 8.4 g) and duodenal ulcer rats with ginkgo (34.0 ± 4.5 g) during the second week. However, weight gain did not significantly differ between sham operation rats with and without ginkgo (25.2 ± 7.7 g versus 32.5 ± 8.4 g), and between sham operation rats without ginkgo and duodenal ulcer rats with ginkgo (32.5 ± 8.4 g versus 34.0 ± 4.5 g).
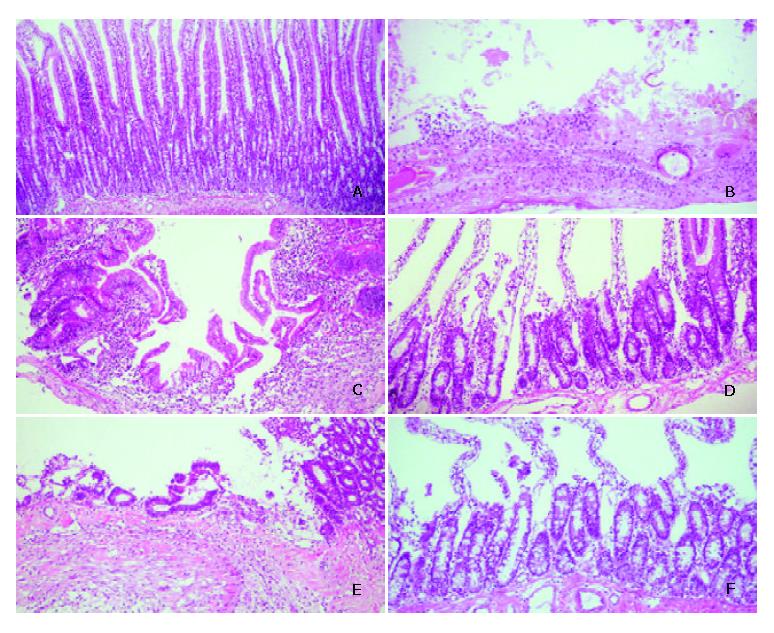
To identify the formation and healing of duodenal ulcer in rats after operation, gross morphological appearance magnified by × 100 or × 200 is shown in Figure 2. Compared with sham operation rats (Figure 2A), duodenal ulcer rats on day 1 before treatment had the discontinuous lining of the mucosal and submucosal layers, damage in the muscular layer, and serious inflammation with aggregation of the leukocytes (Figure 2B), which was similar to human duodenal ulcer in the pathology. The mean diameter of the ulcer damage was 2 mm under microscopic observation. After 7 d treatment, duodenal ulcer rats without ginkgo still had inflammation (Figure 2C). The mean diameter of the ulcer damage reduced to 1 mm. However, the inflammation almost recovered, and the microvilli proliferated in duodenal ulcer rats with ginkgo (Figure 2D). The mean diameter of the ulcer damage reduced to less than 1 mm. After 14 d treatment, the inflammation lessened and the microvilli increased proliferation in duodenal ulcer rats without ginkgo (Figure 2E). The mucosal healing and proliferation were more advanced in duodenal ulcer rats with ginkgo (Figure 2F). The diameter of the ulcer damage was undetectable in duodenal ulcer rats with and without ginkgo.
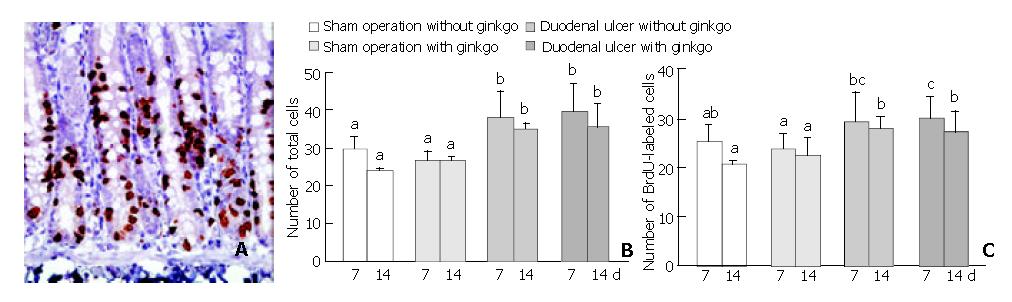
Cell proliferation of the duodenal mucosa was determined by anti-BrdU-immunohistochemical staining (Figure 3A). Duodenal ulcer rats had significantly higher number of total cells (P < 0.05) in the proliferation zone than sham operation rats during the experimental period (Figure 3B). After 7 d treatment, duodenal ulcer rats with ginkgo (30.1 ± 4.1) had significantly higher number of BrdU-labeled cells (P < 0.05) in the proliferation zone compared with sham operation rats with (23.7 ± 2.9) and without ginkgo (25.4 ± 3.0) (Figure 3C). After 14 d treatment, duodenal ulcer rats (27.4 ± 4.0 and 27.8 ± 2.3 for those with and without ginkgo) had higher number of BrdU-labeled cells (P < 0.05) in the proliferation zone than sham operation rats (22.4 ± 3.5 and 20.8 ± 0.5 for those with and without ginkgo). However, the number of both total and BrdU-labeled cells in the proliferation zone did not significantly differ between duodenal ulcer or sham operation rats with and without ginkgo during the experimental period.
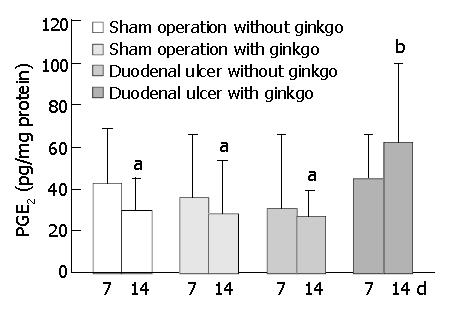
The concentration of PGE2 in the duodenal mucosa did not significantly differ among four groups after 7 d treatment (Figure 4). However, after 14 d treatment, duodenal ulcer rats with ginkgo (61.6 ± 37.5 pg/mg protein) significantly increased PGE2 concentration at least two-fold (P < 0.05) compared with other rats (29.8 ± 14.8 pg/mg, 28.0 ± 25.2 pg/mg, and 26.9 ± 11.9 pg/mg protein for sham operation rats without ginkgo, sham operation rats with ginkgo, and duodenal ulcer rats without ginkgo, respectively). Mucosal PGE2 concentration did not significantly differ among other three groups.
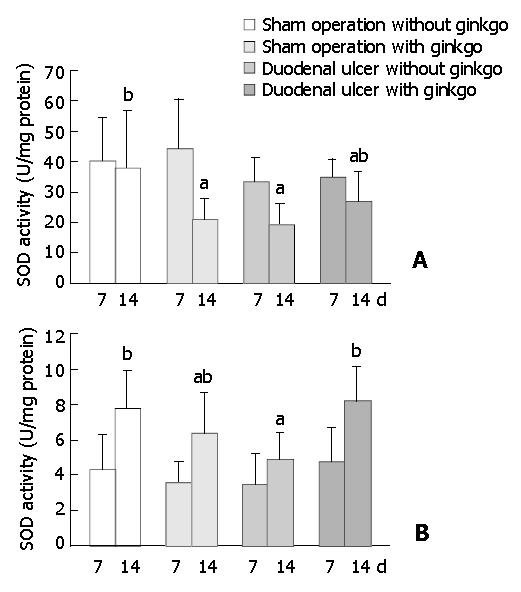
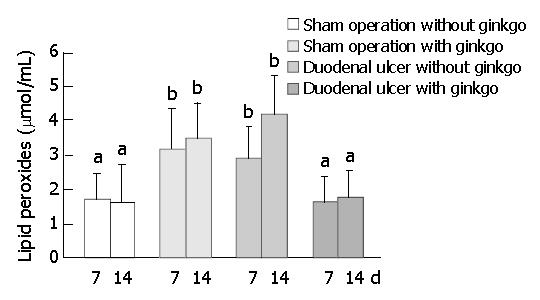
The activity of SOD in the duodenal mucosa and erythrocytes did not significantly differ among four groups after 7 d treatment (Figure 5). After 14 d treatment, sham operation rats with ginkgo (21.0 ± 6.9 U/mg protein) and duodenal ulcer rats without ginkgo (19.4 ± 6.7 U/mg protein) significantly decreased the activity of SOD in the duodenal mucosa by 45% and 49% (P < 0.05), respectively, compared with sham operation rats without ginkgo (38.1 ± 18.9 U/mg protein) (Figure 5A). The activity of SOD in the duodenal mucosa did not significantly differ between duodenal ulcer rats with ginkgo (27.1 ± 9.7 U/mg protein) and other rats. Duodenal ulcer rats without ginkgo (4.87 ± 1.49 U/mg protein) significantly decreased the activity of SOD in the erythrocytes by 37% and 41% (P < 0.05) compared with sham operation rats without ginkgo (7.78 ± 2.16 U/mg protein) and duodenal ulcer rats with ginkgo (8.22 ± 1.92 U/mg protein), respectively (Figure 5B). The activity of SOD in the erythrocytes did not significantly differ between sham operation rats with and without ginkgo (6.31 ± 2.34 U/mg versus 7.78 ± 2.16 U/mg protein), and between sham operation and duodenal ulcer rats with ginkgo (6.31 ± 2.34 U/mg versus 8.22 ± 1.92 U /mg protein). Sham operation rats with ginkgo (3.14 ± 1.21 μmol/mL) and duodenal ulcer rats without ginkgo (2.92 ± 0.90 μmol/mL) significantly increased lipid peroxides in the plasma by 85% and 72% (P < 0.05), respectively, compared with sham operation rats without ginkgo (1.70 ± 0.76 μmol/mL) after 7 d treatment (Figure 6). After 14 d treatment, sham operation rats with ginkgo (3.52 ± 0.99 μmol/mL) and duodenal ulcer rats without ginkgo (4.18 ± 1.12 μmol/mL) significantly increased lipid peroxides in the plasma by 120% and 161% (P < 0.05), respectively, compared with sham operation rats without ginkgo (1.60 ± 1.10 μmol/mL). Duodenal ulcer rats with ginkgo after 7 (1.65 ± 0.69 μmol/mL) and 14 d treatment (1.80 ± 0.73 μmol/mL) significantly decreased lipid peroxides in the plasma by 43% and 57% (P < 0.05), respectively, compared with duodenal ulcer rats without ginkgo in the corresponding days. Lipid peroxidation in the plasma did not significantly differ between duodenal ulcer rats with ginkgo and sham operation rats without ginkgo during the experimental period.
Ginkgo biloba extract was administered by iv injection in this study. The precise dose of ginkgo was well controlled by iv injection into the rats, although oral administration was more convenient for humans. The dose of ginkgo administration was constant at 0.5 mg/(kg·d). However, the total dose was higher in duodenal ulcer rats for 14 d treatment, because weight gain was higher in duodenal ulcer rats with ginkgo after 14 d treatment.
Reactive oxygen species (ROS) could play an important role in the pathogenesis and aggravation of duodenal ulceration[3,11]. Certain forms of ROS such as H2O2 might act as signal transduction messengers to activate transcription factors NF-κB and AP-1, which bind to the promoter region of a large variety of genes that are directly involved in the pathogenesis of diseases[12]. Additionally, oxygen free radicals have been reported to trigger leukocyte adhesion and activation through modulating the expression of leukocyte adhesion molecules and CD markers[13]. Pathological results in our study showed that Ginkgo biloba extract could eliminate inflammation caused by duodenal ulcer probably because of its antioxidant property. Ginkgo biloba extract or its component exerted an anti-inflammatory effect by suppressing the production of active oxygen and nitrogen species[14,15]. Additionally, gross appearance found that more microvilli formation occurred in duodenal ulcer rats with ginkgo compared with those without ginkgo, indicating Ginkgo biloba extract could improve mucosal repair and proliferation. However, semiquantitation of cell proliferation demonstrated that only duodenal ulcer per se significantly increased numbers of total and BrdU-labeled cells in the proliferation zone of the duodenal mucosa. Ginkgo biloba extract did not influence these numbers in duodenal ulcer rats. It could be due to a small dose of BrdU (5 mg /kg, ip) and the short exposure time (1 h) to BrdU before sacrifice in our study compared with the administration of BrdU at 100 mg/kg (ip) 90 min prior to euthanasia in a previous study[16]. It can not be ruled out the possibility that duodenal ulcer rats with ginkgo increase numbers of total and BrdU-labeled cells in the proliferation zone compared with those without ginkgo after administration of BrdU at a higher dose for a longer incorporation time. Dissimilar to this in vivo study, our in vitro study showed that EGb 761 at 1000 mg/L significantly decreased cell proliferation to 45% and 39% of the control group in human hepatocellular carcinoma HepG2 and Hep3B cells[17].
Prostaglandins E, F, and I have been found to exist in the mucosa and fluid of the gastrointestinal tract, and play important roles in modulating the mucosal integrity and various functions of the gastrointestinal tract[18-20]. Besides the antisecretory property of prostaglandins[21], several mechanisms of the gastroduodenal cytoprotective action for PGE2 have been reported, including against disruption of gastric mucosal microvasculature[22], stimulation of mucus and bicarbonate secretion[23], elevation of blood flow[18,24], stimulation of calcium efflux[25], and stabilization of microtubules[25]. Although PGE2 concentration in the duodenal mucosa did not significantly differ among duodenal ulcer rats without ginkgo, sham operation rats with and without ginkgo during the experimental period, duodenal ulcer rats with ginkgo significantly increased PGE2 concentration in the duodenal mucosa by 2.3-fold compared with those without ginkgo after 14 d treatment. Similarly, a previous study found that PGE2 concentration in the duodenal mucosa significantly increased by 2- or 3.2-fold, respectively, after H2-blocker drug treatment with famotidine (40 mg daily) or ranitidine (150 mg twice daily) in duodenal ulcer patients for 4 weeks[26]. The data suggest that Ginkgo biloba extract has similar effectiveness in increasing PGE2 concentration in the duodenal mucosa to H2-blocker drugs. Moreover, increased PGE2 concentration in the duodenal mucosa may play an important role in duodenal cytoprotection to improve the repair of the duodenal mucosa.
Duodenal ulcer rats without ginkgo significantly decreased the activity of SOD in the duodenal mucosa and erythrocytes compared with sham operation rats without ginkgo after 14 days. Similarly, SOD activity markedly depleted in the ulcer edge of patients with duodenal ulcer compared with that in biopsies from the duodenal bulb of healthy control subjects, and increased after one month of anti-ulcer treatment[27]. However, SOD activity in erythrocytes and serum did not differ between patients with duodenal ulcer and healthy control subjects. The decreased activity of SOD might attribute to the increased consumption of SOD for the defense of superoxide radicals generated in the duodenal ulcer rats. After 14 d treatment, Ginkgo biloba extract significantly restored SOD activity in erythrocytes of duodenal ulcer rats, but did not affect SOD activity in duodenal mucosa. The previous study demonstrated that coadministration of SOD and catalase protected against gastric mucosa lesions induced by water immersion restraint stress in rats, and suggested that the protective effect of SOD and catalase could be due to their antioxidant activity to scavenge ROS[28]. Ginkgo biloba extract has been reported as a strong scavenger for superoxide anion[2]. It is assumed that Ginkgo biloba extract given by intravenous injection may directly compensate the utilization of SOD in erythrocytes for the defense of superoxide anion. Whereas the trend for increased SOD activity in the duodenal mucosa of duodenal ulcer rats with ginkgo was not obvious as observed in erythrocytes, probably because the amount of ginkgo reached to the duodenal mucosa was less than that in the circulation, and the treatment duration was not long enough to significantly raise SOD activity in the duodenal mucosa.
Duodenal ulcer rats without ginkgo significantly increased plasma lipid peroxides compared with sham operation rats without ginkgo during the experimental period, suggesting that duodenal ulcer may be accompanied by oxidative damage to the body. The decreased SOD activity in erythrocytes of duodenal ulcer rats without ginkgo might be attributed to the increased utilization of SOD for the protection against the increased lipid peroxides in plasma. Ginkgo biloba extract significantly decreased the elevated lipid peroxides in plasma of duodenal ulcer rats after 7 and 14 d treatment. Similarly, Ginkgo biloba extract has been demonstrated to act as an antioxidant and a free radical scavenger to reduce the increased lipid peroxides induced by gentamicin in rat plasma and kidney[29]. Unexpectedly, sham operation rats with ginkgo had lower SOD activity in the duodenal mucosa and higher lipid peroxides in plasma compared with those without ginkgo. In contrast with Ginkgo biloba extract as an antioxidant in previous studies[2,14,29], it might play a prooxidant role in sham operation rats. Flavonoids with phenol rings in Ginkgo biloba extract could be metabolized by peroxidase to form prooxidant phenoxyl radicals which, in some cases, were sufficiently reactive to cooxidize glutathione or NADH accompanied by extensive oxygen uptake and reactive oxygen species formation[30]. However, no report has directly pointed out the possibility of Ginkgo biloba extract as a prooxidant.
Overall, duodenal ulcer rats with ginkgo had less inflammation and more microvilli proliferation, and significantly reduced lipid peroxides in plasma compared with those without ginkgo after 7 d treatment. Additionally, duodenal ulcer rats with ginkgo tended to increase PGE2 level in the duodenal mucosa and SOD activity in erythrocytes compared with those without ginkgo, but not statistically significant. After 14 d treatment, besides further improvement in the healing of duodenal ulcer observed by gross appearance and a decrease in plasma lipid peroxides, duodenal ulcer rats with ginkgo significantly increased weight gain, PGE2 concentration in the duodenal mucosa, and SOD activity in erythrocytes. Changes in the level of plasma lipid peroxides seemed to be more sensitive and were accompanied by the repair of the duodenal mucosa in duodenal ulcer rats after Ginkgo biloba extract treatment. In conclusion, Ginkgo biloba extract can increase PGE2 level, SOD activity, and reduce oxidative damage by the actions of cytoprotection and antioxidation to improve the repair of the duodenal mucosa in duodenal ulcer rats.
Edited by Wang XL Proofread by Zhu LH
| 1. | Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 327] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Gardès-Albert M, Ferradini C, Sekaki A, Droy-Lefaix MT. Oxy-gen-centered free radicals and their interactions with EGb 761 or CP 202. Advances in Ginkgo biloba extract research: Ginkgo biloba extract (EGb 761) as a free-radical scavenger. New York: Elsevier Science 1993; 1-11. |
| 3. | Zhang Q, Dawodu JB, Etolhi G, Husain A, Gemmell CG, Russell RI. Relationship between the mucosal production of reactive oxygen radicals and density of Helicobacter pylori in patients with duodenal ulcer. Eur J Gastroenterol Hepatol. 1997;9:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Takeuchi K, Nishiwaki H, Niida H, Ueshima K, Okabe S. Duodenal ulcers induced by diethyldithiocarbamate, a superoxide dismutase inhibitor, in the rat: role of antioxidative system in the pathogenesis. Jpn J Pharmacol. 1991;57:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Chen SH, Pan S, Okita K, Takemoto T. Role of oxygen-derived free radicals in the mechanism of cysteamine-induced duodenal ulcer in rats. J Formos Med Assoc. 1994;93:11-14. [PubMed] |
| 6. | Kedziora-Kornatowska K, Tkaczewski W, Blaszczyk J, Buczyn'ski A, Chojnacki J, Kedziora J. Oxygen metabolism in blood of patients with gastric and duodenal ulcer disease. Hepatogastroenterology. 1995;42:246-249. [PubMed] |
| 7. | D'Souza RS, Dhume VG. Gastric cytoprotection. Indian J Physiol Pharmacol. 1991;35:88-98. [PubMed] |
| 8. | Chao JC, Liu KY, Chen SH, Fang CL, Tsao CW. Effect of oral epidermal growth factor on mucosal healing in rats with duodenal ulcer. World J Gastroenterol. 2003;9:2261-2265. [PubMed] |
| 9. | Yamaguchi T, Nakajima N, Kuwayama H, Ito Y, Iwasaki A, Arakawa Y. Gastric epithelial cell proliferation and apoptosis in Helicobacter pylori-infected mice. Aliment Pharmacol Ther. 2000;14 Suppl 1:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 11. | Sieroń A, Kawczyk-Krupka A, Gadowska-Cicha A. [The role of free radicals in inflammatory states, ulceration, and ulcers of the stomach and duodenum]. Pol Merkur Lekarski. 2001;10:113-116. [PubMed] |
| 12. | Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709-720. [PubMed] |
| 13. | Fraticelli A, Serrano CV, Bochner BS, Capogrossi MC, Zweier JL. Hydrogen peroxide and superoxide modulate leukocyte adhesion molecule expression and leukocyte endothelial adhesion. Biochim Biophys Acta. 1996;1310:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Kim HK, Son KH, Chang HW, Kang SS, Kim HP. Inhibition of rat adjuvant-induced arthritis by ginkgetin, a biflavone from ginkgo biloba leaves. Planta Med. 1999;65:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Lim CW, Parker HM, Vesonder RF, Haschek WM. Intravenous fumonisin B1 induces cell proliferation and apoptosis in the rat. Nat Toxins. 1996;4:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Chao JC, Chu CC. Effects of Ginkgo biloba extract on cell proliferation and cytotoxicity in human hepatocellular carcinoma cells. World J Gastroenterol. 2004;10:37-41. [PubMed] |
| 18. | Dajani EZ, Agrawal NM. Protective effects of prostaglandins against nonsteroidal anti-inflammatory drug-induced gastrointestinal mucosal injury. Int J Clin Pharmacol Res. 1989;9:359-369. [PubMed] |
| 19. | Wallace JL, Tigley AW. Review article: new insights into prostaglandins and mucosal defence. Aliment Pharmacol Ther. 1995;9:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Takeuchi K, Kato S, Tanaka A. [Gastrointestinal cytoprotection by prostaglandin E and EP receptor subtypes]. Nihon Yakurigaku Zasshi. 2001;117:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Johansson C, Bergström S. Prostaglandin and protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1982;77:21-46. [PubMed] |
| 22. | Morris GP. Prostaglandins and cellular restitution in the gastric mucosa. Am J Med. 1986;81:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Sugamoto S, Kawauch S, Furukawa O, Mimaki TH, Takeuchi K. Role of endogenous nitric oxide and prostaglandin in duodenal bicarbonate response induced by mucosal acidification in rats. Dig Dis Sci. 2001;46:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yan CD, Gu L, Tian SP, Chen QS, Dai YL, Li DS. [Effects of gastric mucosal blood flow (GMBF) on the role of adaptive cytoprotection of rat gastric mucosa]. Shengli Xuebao. 1996;48:469-476. [PubMed] |
| 25. | Banan A, Smith GS, Deshpande Y, Rieckenberg CL, Kokoska ER, Miller TA. Prostaglandins protect human intestinal cells against ethanol injury by stabilizing microtubules: role of protein kinase C and enhanced calcium efflux. Dig Dis Sci. 1999;44:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Lezoche E, Vagni V, D'Alessandro MD, Mariani P, Carlei F, Lomanto D, Nardovino M, Martelli A, Speranza V. Action of famotidine and ranitidine on prostaglandin E2 (PGE2) content of fundic and duodenal mucosa in duodenal ulcer patients. Drugs Exp Clin Res. 1987;13:655-658. [PubMed] |
| 27. | Klinowski E, Broide E, Varsano R, Eshchar J, Scapa E. Superoxide dismutase activity in duodenal ulcer patients. Eur J Gastroenterol Hepatol. 1996;8:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Ohta Y, Nishida K. Protective effect of coadministered superoxide dismutase and catalase against stress-induced gastric mucosal lesions. Clin Exp Pharmacol Physiol. 2003;30:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Naidu MU, Shifow AA, Kumar KV, Ratnakar KS. Ginkgo biloba extract ameliorates gentamicin-induced nephrotoxicity in rats. Phytomedicine. 2000;7:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Galati G, Sabzevari O, Wilson JX, O'Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |