Published online Feb 15, 2004. doi: 10.3748/wjg.v10.i4.491
Revised: July 8, 2003
Accepted: July 24, 2003
Published online: February 15, 2004
AIM: To examine the expression of nuclear factor kappaB (NF-κB) and its target genes in intestinal metaplasia (IM), dysplasia (DYS) and gastric carcinoma (GC) infected with Helicobacter pylori (H pylori) and to investigate the mechanism underlying H pylori cytotoxin associated gene A (cag A) infection leading to gastric adenocarcinoma.
METHODS: Expressions of NF-κB/p65 and its target genes: c-myc, cyclinD1 and bcl-xl were immunohistochemically examined in 289 cases of gastric biopsy and resection specimens from patients with IM, DYS and GC infected with H pylori. H pylori in the above mentioned tissues was detected by Warthin-Starry stain and rapid urease tests. IgG antibody to cagA in sera of the patients was measured by ELISA.
RESULTS: The positive rates of NF-κB/p65 were significantly higher in groups with cagA of IMI-II(28/33), IM III(48/52), DYSI(27/31), DYS II-III(28/32), GC(35/40) than in groups without cagA of IMI-II(4/17), IMIII(3/20), DYSI(3/20), DYSII-III(6/21), GC(10/23). The expressions of c-myc, cyclinD1, and bcl-xl were significantly higher in groups with cagA of IM III(47/52, 49/52, 46/52), DYSII-III(29/32, 26/32, 25/32) than in groups without cagA of IM III(8/20, 7/20, 5/20), DYSII-III(10/21, 8/21, 3/21), which were in conformity with the expression of NF-κB in IM III, and DYSII-III. A significantly higher expression level of NF-κB/p65, c-myc, cyclinD1 and bcl-xl was detected in intestinal type GC(27/28, 18/28, 22/28, 24/28) than in diffuse type GC(8/12, 3/12, 3/12, 6/12), respectively.
CONCLUSION: There may be two different molecular mechanisms in the occurrence of intestinal and diffuse type gastric carcinomas. Intestinal type gastric carcinoma is strongly associated with high expression of c-myc, cyclinD1 and bcl-xl through NF-κB/p65 activated by H pylori cagA. Inhibiting the activity of NF-κB is an effective and promising way to prevent intestinal type gastric carcinoma.
- Citation: Yang GF, Deng CS, Xiong YY, Gong LL, Wang BC, Luo J. Expression of nuclear factor-kappa B and target genes in gastric precancerous lesions and adenocarcinoma: Association with Helicobactor pylori cagA (+) infection. World J Gastroenterol 2004; 10(4): 491-496
- URL: https://www.wjgnet.com/1007-9327/full/v10/i4/491.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i4.491
A close link between gastric carcinoma and H pylori infection has been found[1-6]. Animal models of gastric carcinoma induced by H pylori provide strong evidence[7-10]. There is a close association between H pylori cagA infection and non-cardio gastric carcinoma[11-13]. However, the exact mechanism of H pylori cagA leading to gastric carcinoma remains indistinct.
NF-κB is a sequence-specific transcription factor known to be involved in inflammatory and immune responses. A wide variety of genes regulated by NF-κB includes those encoding cytokines, antimicrobial peptides, adhesion molecules[14]. Recently, it has been found that NF-κB contributes to the activation of genes encoding regulators of anti-apoptosis and cell proliferation. Hence, the role of NF-κB in tumorigenesis has attracted increasing attention. It is well documented that cagA of H pylori can activate NF-κB and H pylori cagA infection often causes chronic atrophic gastritis accompanied by intestinal metaplasia and dysplasia. NF-κB is not only involved in inflammatory responses but also related to carcinogenesis[15-18]. We deduce that NF-κB may be an important link between these two processes. This study was to investigate the possible association between H pylori cagA infection and the expression of NF-κB/p65 and its target genes in GC. Warthin-Starry staining and rapid urease tests were performed to detect H pylori. ELISA was employed to measure IgG antibody to cagA. HID/AB/PAS staining method was used to distinguish sulphates, neutral and acid mucin. Immunohistochemical techniques were employed to determine the expression of NF-κB/p65 and its target genes in specimens of IM, DYS, and GC.
A total of 289 cases were recruited from patients suffering from gastritis who underwent endoscopic examination and biopsy and from gastric cancer patients received surgical resections from 2001 to 2002 at Zhongnan Hospital, Wuhan. Among the 289 cases, 179 were male and 110 were female. The average age was 48.5 years (range, 19-75 years). From each patient four samples were taken from gastric antrum and corpus, respectively. Rapid urease tests were done first. Patients who obtained positive results had their blood samples taken, which were stored at -20 °Cuntil ELISA assay. No patients had received chemotherapy or radiation therapy before surgery. Samples were fixed in 10% neutrally buffered formalin for histological, histochemical and immunohistochemical studies.
For histological evaluation, formalin-fixed tissues were embedded in paraffin, cut into 4 μm thick sections and stained with H&E. GC was histologically divided into early stage and advanced stage, with or without lymph node metastasis, intestinal type and diffuse type[19].
Intestinal metaplasia was classified into three sub-types according to both morphological and histochemical criteria. Dysplasia was divided into three grades[20-21].
The presence of serum anti-cagA IgG antibody was tested with commercial enzyme-linked immunosorbent assay (ELISA) kits (Jing Ying Biotechnology Company, Shanghai). Briefly, a recombinant protein fragment of cagA, a purified form of Escherichia coli cell lysates, was used as an antigen and fixed to a 96-well plate in carbonate-bicarbonate buffer. After incubation of treated wells with serum diluted 1:100, alkaline phosphatase-conjugated goat anti-human IgG was added. After addition of phosphatase substrate, absorbance was read at 405 nm. Based on the results from H pylori-negative controls, a value of ≥ 0.18 was considered to be positive of IgG antibodies.
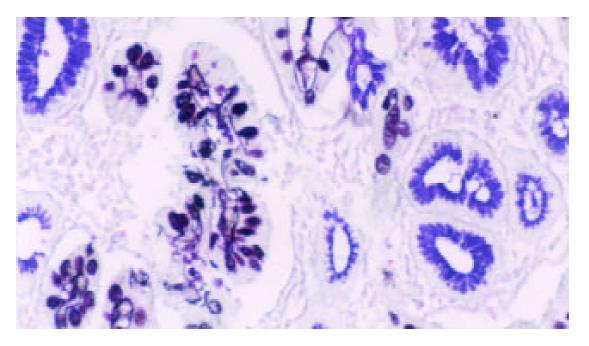
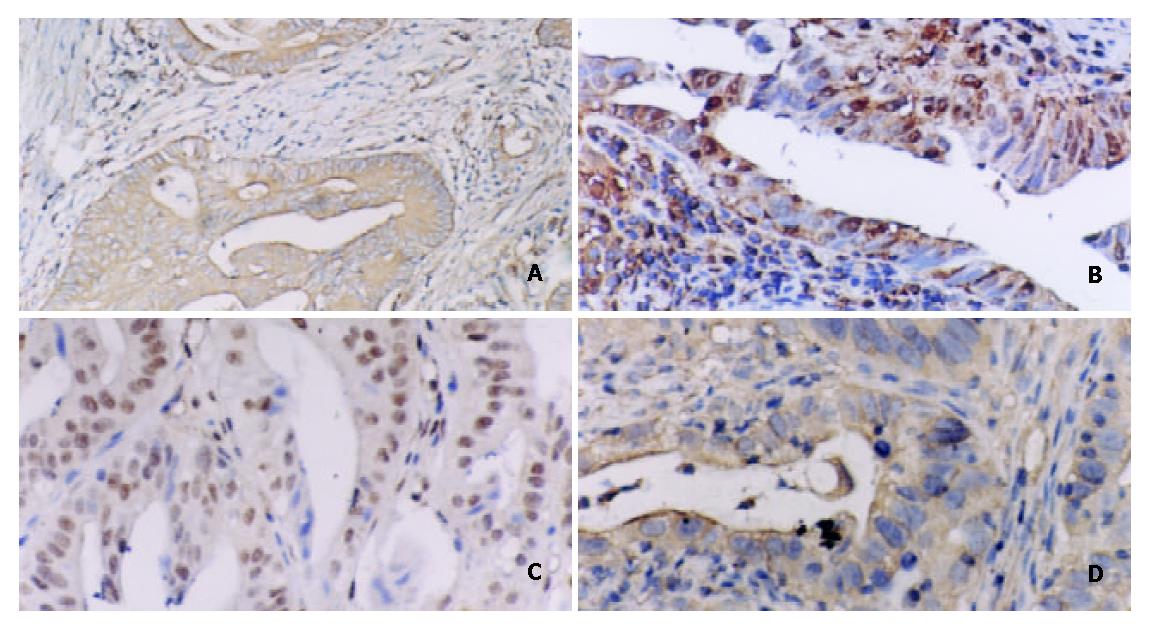
Mucin histochemical studies were performed using HID/AB/PAS staining described by Walsh et al[22] and Xin et al[23]. IM I (complete type) showed a positive staining of AB (blue color). IM II (incomplete small intestinal type) had PAS (magenta color) and AB positive staining. IM III (incomplete colonic metaplasia) had both positive A B (blue color) and HID staining (sulphomucins brown in color) (as shown in Figure 1).
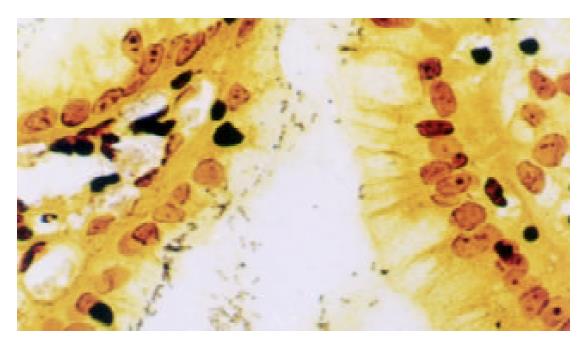
Warthin-Starry stain was carried out using standard histological laboratory methods. Positive H pylori showed dark-brown-stained bacillary structures in epithelium of mucosa or in glandular recess (as shown in Figure 2). Samples of positive ELISA and rapid urease test or Warthin-Starry stain were considered to be H pylori cagA positive. Samples of positive rapid urease test and Warthin-Starry stain were considered to be H pylori positive.
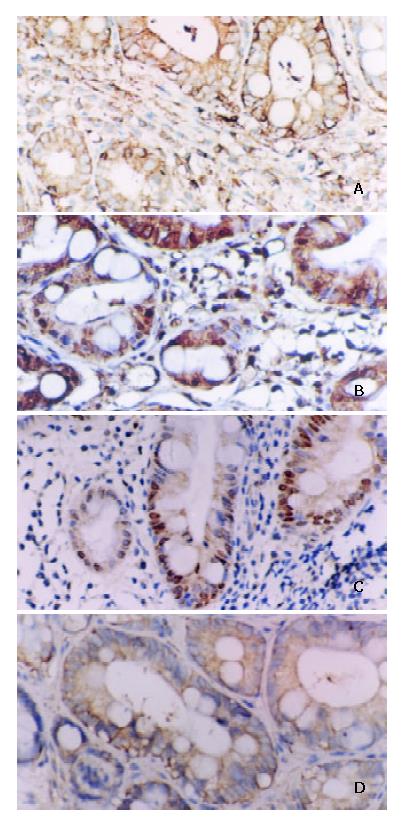
Serial 4 μm thick sections were made and mounted on poly L-lysine coated slides. Paraffin sections were immersed in xylene for 5 minutes and hydrated using a gradient series of alcohol. Antigen retrieval was routinely performed by immersing the sections in citric acid buffer (pH6.0), in a microwave oven for 15 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min and then incubated with a primary antibody in a humidified chamber at 4 °Covernight. Primary antibody was monoclonal mouse anti-NF-κB/p65 antibody (Santa Cruze) at 1:200 dilution. Monoclonal mouse anti-cyclinD1, c-myc antibody (Maixin Biotechnology Company), monoclonal mouse anti-bcl-xl antibody (Beijing Zhongsheng Biotechnology Company) and ultrasensitive SP kit were obtained from the above-mentioned two companies. Sections were counterstained for 5 min with Delafield’s haematoxylin and then mounted. Human colon cancer having infiltration of lymphatic cells with intense staining for NF-κB, c-myc, cyclinD1, bcl-xl was used as a positive control. As a negative control, PBS was used instead of primary antibodies. Expressions of NF-κB, c-myc, cyclinD1, bcl-xl in carcinoma cells, metaplastic and dysplastic epithelia were evaluated according to the ratio of positive cells and defined as negative (0% - 9%) and positive (10% - 100%). Positive cells of NF-κB/p65 mainly showed a plasmatic staining (Figure 3). Positive cells of c-myc, cyclin D1 showed nuclear and plasmatic staining and bcl-xl showed a plasmatic staining (Figure 3).
Differences among groups were evaluated by using Chi-square test and Fisher’s exact test. Differences were considered to be statistically significant at P < 0.05.
The highest positive rates of NF-κB/p65 and its target genes were in IM III among IM I-II, IM II, DYS I, DYS II-III and GC, but there were no significant differences among them (Table 1). The expression of NF-κB/p65 was significantly higher in groups with H pylori cagA of IMI-II(28/33), IM III(48/52), DYSI(27/31), DYSII-III(28/32), GC(35/40) than in groups without H pylori cagA of IMI-II(4/17), IM III(3/20), DYSI(3/20), DYSII-III(6/21), GC(10/23) as shown in Table 2. The expression of c-myc, cyclinD1, bcl-xl was significantly higher in groups with cagA of IM III(47/52, 49/52, 46/52), DYSII-III(29/32, 26/32, 25/32) than in groups without cagA of IM III(8/20, 7/20, 5/20), DYSII-III(10/21, 8/21, 3/21) (Figure 4), which was conformable to the expression of NF-κB in IM III, DYSII-III, as shown in Table 2.
| n | NF-κB/p65 positive(n) | % | C-myc positive (n) | % | CyclinD1 positive (n) | % | Bcl-xl positive (n) | % | |
| IM I-II | 50 | 32 | 64.00 | 35 | 70.00 | 34 | 68.00 | 33 | 66.00 |
| IM III | 72 | 51 | 70.83 | 55 | 76.39 | 56 | 77.78 | 51 | 70.83 |
| DYS I | 51 | 33 | 64.71 | 36 | 70.59 | 35 | 68.63 | 34 | 66.67 |
| DYS II-III | 53 | 34 | 64.15 | 39 | 73.58 | 34 | 64.15 | 28 | 52.83 |
| GC | 63 | 45 | 71.43 | 33 | 52.38 | 37 | 58.73 | 44 | 69.84 |
| CagA (n) | NF-κB/p65 positive(n) | χ2 | Exact | C-myc posotive(n) | χ2 | Exact | CyclinD1 positive(n) | χ2 | Exact | Bc1-x1 positive(n) | χ2 | Exact | |
| IM I-II | +33 | 28 | 18.311 | 0.000 | 22 | 0.514 | 0.533 | 21 | 0.849 | 0.524 | 21 | 0.242 | 0.757 |
| -17 | 4 | 13 | 13 | 12 | |||||||||
| IM III | +52 | 48 | 41.785 | 0.000 | 47 | 20.331 | 0.000 | 49 | 29.319 | 0.000 | 46 | 28.158 | 0.000 |
| -20 | 3 | 41.785 | 8 | 7 | 5 | ||||||||
| Dys I | +31 | 27 | 0.000 | 22 | 0.005 | 1.000 | 22 | 0.201 | 0.760 | 21 | 0.041 | 1.000 | |
| -20 | 3 | 26.089 | 14 | 13 | 13 | ||||||||
| Dys II-III | +32 | 28 | 0.000 | 29 | 12.065 | 0.001 | 26 | 10.268 | 0.001 | 25 | 20.736 | 0.000 | |
| -21 | 6 | 19.154 | 10 | 8 | 3 | ||||||||
| GC | +40 | 35 | 0.000 | 21 | 0.128 | 0.721 | 25 | 0.642 | 0.432 | 30 | 1.384 | 0.239 | |
| -23 | 10 | 13.867 | 11 | 12 | 14 |
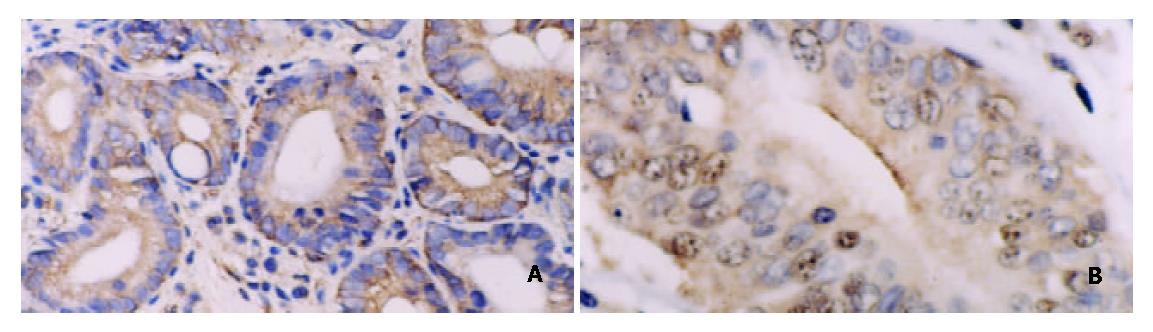
The expression of NF-κB/p65, c-myc, cyclinD1, and bcl-xl was significantly higher in intestinal typeGC(27/28, 18/28, 22/28, 24/28) than in diffuse type GC(8/12, 3/12, 3/12 and 6/12), respectively (Figure 5), as shown in Table 3. It was also observed that the expression of NF-κB/p65, c-myc was higher in advanced GC (26/27, 18/27) than in early stage GC(9/13, 3/13) (P < 0.05). The expression of c-myc was higher in groups with lymph node metastasis(19/30) than in groups without lymph node metastasis (2/10) (P < 0.05) (Table 3).
| Clinicopathology | n | NF-κB/p65 positive (n) | χ2 | P | C-myc positive (n) | χ2 | P | CyclinD1 positive (n) | χ2 | P | Bcl-xl positive (n) | χ2 | P |
| Early stage | 13 | 9 | 5.877 | 0.031 | 3 | 6.687 | 0.017 | 7 | 0.615 | 0.498 | 5 | ||
| Advanced stage | 27 | 26 | 18 | 18 | 10 | 0.008 | 1.000 | ||||||
| Intestinal type | 28 | 27 | 6.803 | 0.022 | 18 | 5.199 | 0.038 | 22 | 10.286 | 0.003 | 24 | ||
| Diffuse type | 12 | 8 | 3 | 3 | 6 | 5.714 | 0.041 | ||||||
| Metastasis of LN | 30 | 26 | 0.076 | 1.000 | 19 | 5.647 | 0.028 | 16 | 4.302 | 0.060 | 22 | ||
| No metastasis of LN | 10 | 9 | 2 | 9 | 8 | 0.178 | 1.000 |
We also observed the expression of NF-κB/p65, c-myc, cyclinD1 in inflammatory cells of gastric lamina propria and stroma, and 5.6% nuclear staining of NF-κB/p65 was in the groups with H pylori cagA in the tissues of IM, DYS and GC.
NF-κB was first identified as a regulator of expression of the kappa light chain gene in murine B lymphocytes but has subsequently been found in many different cells[14]. NF-κB is made up of protein dimers that bind to a common sequence motif known as the κB site. The activated form of NF-κB is a heterodimer, which usually consists of two proteins: a p65 subunit and a p50 subunit. In unstimulated cells, NF-κB is found in cytoplasm and is bound to IκB, which prevents it from entering nuclei. When cells are stimulated, specific kinase phosphorylate IκB results in IκB’s rapid degradation by proteasomes. The release of NF-κB from IκB would result in passage of NF-κB into nuclei, where it binds to specific sequences in promoter regions of target genes and further activates target genes[24-28].
H pylori cagA is one of the virulent strains of H pylori. Studies have demonstrated that activation of NF-κB by H pylori requires genes in cag PAI (pathogenicity island). CagA protein of H pylori PAI can be translocated into gastric epithelial cells by a type IV secretion system encoded by cagPAI and is tyrosine-phosphorylated. Tyrosine phosphorylated cagA could induce changes in the tyrosine phosphorylation status of distinct cellular proteins and further activate NF-κB[29-32]. In the present study, we found that high expression of NF-κB/p65 was detected in the tissues of H pylori cagA group with metaplasia, dysplasia and gastric carcinoma. We also observed that expression of NF-κB/p65 was mainly localized in cytoplasm. Although nuclear localization of NF-κB was considered as equivalent to NF-κB activation, it showed 5.6% positive rate in H pylori cagA group in our study, which was similar to that of Nakayama et al[33]. Though it seems difficult to determine whether immunohistological detection of a certain protein reflects its dynamic localization, we believe that large amounts of NF-κB/p65 in the cytoplasm of tissues infected with H pylori cagA may be easily activated by cagA of H pylori, even by a slight stimulation of cagA protein. NF-κB/p65 activated by cagA up-regulated the expression of target genes in IM, DYS and GC, and might play an important role in the occurrence and development of gastric carcinoma caused by H pylori cagA infection. As one of the NF-κB target genes[34,35], bcl-xl belongs to the anti-apoptosis gene family and exerts its anti-apoptosis effect by inhibiting the release of apoptosis inducing factors.
Some investigations showed that tumor cells with activated NF-κB were not sensitive to radio and chemotherapeutic agents, while inhibition of NF-κB activity could enhance the radio- and chemosensitivity of cancer treatment[36,37].
Activated NF-κB has also been shown to directly stimulate the transcription of genes that encode c-myc and cyclinD1 because a kB binding site is present within the c-myc, and cyclinD1 promoter[16,38,39,40]. c-myc is known to be a strong inducer of proliferation and is believed to be critical for the oncogenic properties. Studies showed that aberrant c-myc expression was widespread in tumor cells and important for multistep carcinogenesis[41,42]. However, proliferation induced by c-myc requires the concomitant mutant of p53. In gastric precancerous lesions infected with H pylori, mutants of p53 were often confirmed[43,44]. cyclinD1 is a cell cycle regulatory gene. It is essential for G1 phase progression and has been implicated in the pathogenesis of several human malignancies, including gastric carcinoma[45-47].
As evidenced here, high expressions of c-myc, cyclinD1, bcl-xl concomitant with NF-κB/p65 in the tissues of cagA+ of IM III and DYS II-III could drive cells inappropriately through the cell cycle to evade apoptosis, resulting in uncontrolled proliferation characteristic of neoplastic cells. In addition, weak to moderate staining of NF-κB/p65, c-myc, cyclinD1 of inflammatory cells in the stroma of gastric lesions was observed. The above results, taken together, could be explained in two ways. First, H pylori cagA infection led to gastritis, and activated NF-κB. Activated NF-κB could promote inflammatory cells and epithelium of gastritis to produce large amounts of reaction oxygen species (ROS), cyclooxygenase-2 (COX-2), IL-6, and IL-8[48-53]. ROS played an important role in damaging DNA of epithelium of gastric lesions[54,55]. COX-2 could suppress epithelial cell apoptosis leading to cell proliferation. Some studies showed that overexpression of COX-2 had a strong association with gastric carcinoma[56,57]. IL-6, and IL-8 could directly induce proliferation of epithelium[58,59]. Second, cagA of H pylori activated NF-κB in IM III and DYS II-III. Activated NF-κB up-regulated the expression of c-myc, cyclinD1, bcl-xl, and prevented the death of epithelial cells that had undergone chromosomal rearrangements or other types of DNA damage. c-myc, cyclinD1 enhanced the proliferation of epithelial cells with mutant genes, leading to gastric carcinoma. Such cells are normally eliminated by means of checkpoint control, such as the p53 pathway. As previously mentioned, mutated p53 was often found in precancerous lesions of gastritis infected with H pylori cagA. The present study showed that the expression of NF-κB/p65, c-myc, cyclinD1 and bcl-xl was significantly higher in intestinal type carcinoma than in diffuse type carcinoma, suggesting that the occurrence of the two types of gastric adenocarcinomas may have different molecular mechanisms, which is similar to other author’s viewpoint[60,61]. H pylori cagA infection can lead to intestinal type gastric carcinoma which is strongly associated with the up-regulation of c-myc, cyclinD1 and bcl-xl through NF-κB. Moreover, the expression of NF-κB and c-myc was higher in advanced stage than in early stage GC. There was no relationship between stages of gastric adenocarcinoma and expression of c-myc, cyclinD1, while higher expression of c-myc was detected in the group with lymph mode metastasis as compared with the group without. It is concluded that high expression of NF-κB/p65 and its target genes in H pylori cagA group of IM III, DYS II-III and intestinal type GC may play an important role in various stages of H pylori cagA induced intestinal type gastric carcinoma. In GC infected with H pylori cagA, the expression of NF-κB/p65, c-myc is associated with infiltration of gastric carcinoma. Moreover, expression of c-myc is linked with lymph node metastasis of gastric carcinoma.
Our results suggest that there may be two different molecular mechanisms in the development of intestinal and diffuse type gastric carcinomas. Intestinal type gastric carcinoma is associated with the high expression of c-myc, cyclinD1 and bcl-xl through NF-κB/p65 activated by H pylori cagA. A better understanding of the molecular mechanisms would contribute to the prevention and prognosis of gastric carcinoma.
Edited by Zhu LH and Wang XL
| 1. | Krejs GJ. [Helicobacter pylori and stomach cancer]. Acta Med Austriaca. 2000;27:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Gschwantler M, Dragosics B. [Physiopathology of Helicobacter pylori infections]. Acta Med Austriaca. 2000;27:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Koop H. [Prevention of gastric cancer: Helicobacter--eradication and immunization]. Praxis (Bern 1994). 1998;87:1233-1235. [PubMed] |
| 4. | Vandenplas Y. Helicobacter pylori infection. World J Gastroenterol. 2000;6:20-31. [PubMed] |
| 5. | Zhuang XQ, Lin SR. Research of Helicobacter pylori infection in precancerous gastric lesions. World J Gastroenterol. 2000;6:428-429. [PubMed] |
| 6. | Zhang ZW, Farthing MJ. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol. 1999;5:369-374. [PubMed] |
| 7. | Yao YL, Xu B, Song YG, Zhang WD. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60-63. [PubMed] |
| 8. | Fujioka T, Honda S, Tokieda M. Helicobacter pylori infection and gastric carcinoma in animal models. J Gastroenterol Hepatol. 2000;15 Suppl:D55-D59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Fujioka T, Murakami K, Kodama M, Kagawa J, Okimoto T, Sato R. Helicobacter pylori and gastric carcinoma--from the view point of animal model. Keio J Med. 2002;51 Suppl 2:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848-854. [PubMed] |
| 11. | Debets-Ossenkopp YJ, Reyes G, Mulder J, aan de Stegge BM, Peters JT, Savelkoul PH, Tanca J, Peña AS, Vandenbroucke-Grauls CM. Characteristics of clinical Helicobacter pylori strains from Ecuador. J Antimicrob Chemother. 2003;51:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Ławniczak M, Starzyńska T. [Helicobacter pylori CagA(+) infection in gastric cancer patients]. Pol Merkur Lekarski. 2002;13:216-220. [PubMed] |
| 14. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3589] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 15. | Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 2963] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 16. | Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1908] [Cited by in RCA: 1956] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 17. | Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 18. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. [PubMed] |
| 19. | Laurén P. Histogenesis of intestinal and diffuse types of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;180:160-164. [PubMed] |
| 20. | Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 250] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 22. | Walsh MD, Jass JR. Histologically based methods for detection of mucin. Methods Mol Biol. 2000;125:29-44. [PubMed] |
| 23. | Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53-59. [PubMed] |
| 24. | Siebenlist U. Signal transduction. Barriers come down. Nature. 2001;412:601-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 26. | Foryst-Ludwig A, Naumann M. p21-activated kinase 1 activates the nuclear factor kappa B (NF-kappa B)-inducing kinase-Ikappa B kinases NF-kappa B pathway and proinflammatory cytokines in Helicobacter pylori infection. J Biol Chem. 2000;275:39779-39785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Naumann M. Nuclear factor-kappa B activation and innate immune response in microbial pathogen infection. Biochem Pharmacol. 2000;60:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856-44864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1343] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 30. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 967] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 31. | Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 308] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Backert S, Müller EC, Jungblut PR, Meyer TF. Tyrosine phosphorylation patterns and size modification of the Helicobacter pylori CagA protein after translocation into gastric epithelial cells. Proteomics. 2001;1:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Mori N, Fujii M, Cheng G, Ikeda S, Yamasaki Y, Yamada Y, Tomonaga M, Yamamoto N. Human T-cell leukemia virus type I tax protein induces the expression of anti-apoptotic gene Bcl-xL in human T-cells through nuclear factor-kappaB and c-AMP responsive element binding protein pathways. Virus Genes. 2001;22:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Chiao PJ, Na R, Niu J, Sclabas GM, Dong Q, Curley SA. Role of Rel/NF-kappaB transcription factors in apoptosis of human hepatocellular carcinoma cells. Cancer. 2002;95:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Cusack JC, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535-3540. [PubMed] |
| 37. | Russo SM, Tepper JE, Baldwin AS, Liu R, Adams J, Elliott P, Cusack JC. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Grumont RJ, Strasser A, Gerondakis S. B cell growth is controlled by phosphatidylinosotol 3-kinase-dependent induction of Rel/NF-kappaB regulated c-myc transcription. Mol Cell. 2002;10:1283-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Park JK, Chung YM, Kang S, Kim JU, Kim YT, Kim HJ, Kim YH, Kim JS, Yoo YD. c-Myc exerts a protective function through ornithine decarboxylase against cellular insults. Mol Pharmacol. 2002;62:1400-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690-2698. [PubMed] |
| 41. | Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int. 2000;50:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Chen JP, Lin C, Xu CP, Zhang XY, Fu M, Deng YP, Wei Y, Wu M. Molecular therapy with recombinant antisense c-myc adenovirus for human gastric carcinoma cells in vitro and in vivo. J Gastroenterol Hepatol. 2001;16:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Murakami K, Fujioka T, Kodama M, Honda S, Okimoto T, Oda T, Nishizono A, Sato R, Kubota T, Kagawa J. Analysis of p53 mutations and Helicobacter pylori infection in human and animal models. J Gastroenterol. 2002;37 Suppl 13:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Teh M, Tan KB, Seet BL, Yeoh KG. Study of p53 immunostaining in the gastric epithelium of cagA-positive and cagA-negative Helicobacter pylori gastritis. Cancer. 2002;95:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Umekita Y, Ohi Y, Sagara Y, Yoshida H. Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer. 2002;98:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Shao JC, Wu JF, Wang DB, Qin R, Zhang H. Relationship between the expression of human telomerase reverse transcriptase gene and cell cycle regulators in gastric cancer and its significance. World J Gastroenterol. 2003;9:427-431. [PubMed] |
| 47. | Yu Z, Wang W. [The expressions of telomerase activity and cyclinD1 protein in laryngeal squamous cell carcinoma]. Linchuang Erbiyanhouke Zazhi. 2002;16:286-288. [PubMed] |
| 48. | Danese S, Cremonini F, Armuzzi A, Candelli M, Papa A, Ojetti V, Pastorelli A, Di Caro S, Zannoni G, De Sole P. Helicobacter pylori CagA-positive strains affect oxygen free radicals generation by gastric mucosa. Scand J Gastroenterol. 2001;36:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2002;368:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Kim JS, Kim JM, Jung HC, Song IS. Expression of cyclooxygenase-2 in human neutrophils activated by Helicobacter pylori water-soluble proteins: possible involvement of NF-kappaB and MAP kinase signaling pathway. Dig Dis Sci. 2001;46:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Tu B, Gong JP, Feng HY, Wu CX, Shi YJ, Li XH, Peng Y, Liu CA, Li SW. Role of NF-kB in multiple organ dysfunction during acute obstructive cholangitis. World J Gastroenterol. 2003;9:179-183. [PubMed] |
| 52. | Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001;36:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Kim H, Lim JW, Seo JY, Kim KH. Oxidant-sensitive transcription factor and cyclooxygenase-2 by Helicobacter pylori stimulation in human gastric cancer cells. J Environ Pathol Toxicol Oncol. 2002;21:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Papa A, Danese S, Sgambato A, Ardito R, Zannoni G, Rinelli A, Vecchio FM, Gentiloni-Silveri N, Cittadini A, Gasbarrini G. Role of Helicobacter pylori CagA+ infection in determining oxidative DNA damage in gastric mucosa. Scand J Gastroenterol. 2002;37:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Smoot DT, Elliott TB, Verspaget HW, Jones D, Allen CR, Vernon KG, Bremner T, Kidd LC, Kim KS, Groupman JD. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis. 2000;21:2091-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Guo XL, Wang LE, Du SY, Fan CL, Li L, Wang P, Yuan Y. Association of cyclooxygenase-2 expression with Hp-cagA infection in gastric cancer. World J Gastroenterol. 2003;9:246-249. [PubMed] |
| 57. | Li HX, Chang XM, Song ZJ, He SX. Correlation between expression of cyclooxygenase-2 and angiogenesis in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:674-677. [PubMed] |
| 58. | Galizia G, Lieto E, De Vita F, Romano C, Orditura M, Castellano P, Imperatore V, Infusino S, Catalano G, Pignatelli C. Circulating levels of interleukin-10 and interleukin-6 in gastric and colon cancer patients before and after surgery: relationship with radicality and outcome. J Interferon Cytokine Res. 2002;22:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Zhang X, Chen Y, Liu Y, Zhou X, Fan D. [Local cytokines profile in gastric cancer lesions]. Zhonghua Zhongliu Zazhi. 2002;24:14-16. [PubMed] |
| 60. | El-Zimaity HM, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Fukuda S, Tanaka M, Soma Y, Shimoyama T, Mikami T, Crabtree JE, Saito H, Munakata A, Yoshida Y. Histological analysis of gastritis and Helicobacter pylori infection in patients with early gastric cancer: a case-control study. J Gastroenterol Hepatol. 2000;15:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |