Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.3026
Revised: December 2, 2003
Accepted: December 22, 2003
Published online: October 15, 2004
AIM: To study changes of inflammation-associated cytokine expressions during early phase of endotoxic shock in macague.
METHODS: Experiments were performed in Macaque mulatta treated with LPS 2.8 mg/kg in shock model group or with normal saline in control group. Blood samples were collected before, or 60 min, or 120 min after LPS injection, respectively. Liver and spleen tissues were obtained at 120 min after LPS injection. The plasma levels of TNF-α , IL-1 β , IL-10 and IL-12P40 were determined by double-antibody sandwich ELISA with antibodies against human cytokines. The mRNA levels of TNF-α , IL-1 β , and IL-18 in peripheral blood mononuclear cells (PBMCs), liver and spleen were examined by real-time fluorescence semi-quantitative RT-PCR with the primers based on human genes.
RESULTS: Mean systemic arterial pressure (MAP), systemic vascular resistance index (SVRI) and left ventricular work index (LVWI) of macaques were significant declined in shock model group on average 60 min after LPS injection. The plasma levels of TNF-α and IL-10 were significantly increased 60 min after LPS injection and then decreased. The plasma levels of IL-1 β and IL-12P40 were significantly increased at 120 min after LPS injection. The mRNA levels of TNF-α and IL-1 β were significantly increased 60 min after LPS stimulation in PBMCs and 120 min after LPS stimulation in livers. The mRNA level of IL-18 was significantly increased 120 min after LPS stimulation in PBMCs and livers. But in spleen, only TNF-α mRNA level in LPS group was significantly higher 120 min after LPS stimulation, compared with that in control group.
CONCLUSION: An endotoxic shock model of Macaque mulatta was successfully established. Both antibodies for ELISA and PCR primers based on human cytokine assays were successfully applied to detect macaque cytokines. In the model, inflammatory cytokines, such as TNF-α , IL-1 β , IL-12 and IL-18 as well as anti-inflammation cytokine IL-10, were released at very early phase of endotoxic shock within 120 min after LPS injection. PBMCs and liver cells might be the important sources of these cytokines.
- Citation: Ji XH, Sun KY, Feng YH, Yin GQ. Changes of inflammation-associated cytokine expressions during early phase of experimental endotoxic shock in macaques. World J Gastroenterol 2004; 10(20): 3026-3033
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/3026.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.3026
Endotoxin-induced shock mostly occurs during serious infection with Gram-negative bacteria. Lipopolysaccharide (LPS), residing in the outer membrane of all Gram-negative bacteria and being the major component of Gram-negative bacteria cell walls and the toxic component of endotoxin, is considered as an important initiating factor of the Gram-negative septic syndrome in human. Gram-negative sepsis has aroused great concern in clinic because of its high mortality. But LPS is not the direct causative factor. It is well known that LPS activates immunocytes, such as monocyte-macrophage and lymphocyte, and induces these cells to excessively release a series of potent inflammatory cytokines, including TNF- α , IL-1 β , IL-6, IL-8 and IL-18. It is the excessive cytokines, such as TNF- α , IL-1 β , IL-6 and IL-18, that result in high fever, hypotension, vascular endothelial cell damage and disseminated intravascular coagulation, blood capillary leak syndrome, and multiple organ failure. On the other hand, in patients with primary liver injury, such as viral hepatitis, LPS plays an important role in intestinal endotoxemia, which in turn induces secondary liver injury and liver failure[1]. New therapeutic concepts for the treatment of endotoxic shock or endotoxemia with anti-inflammatory cytokines have been developed. Although knowledge about the changes of inflammation-associated cytokines during endotoxemia and endotoxic shock has been well addressed, the change pattern of inflammatory cytokines just during the early phase of endotoxemia and endotoxic shock is still not clear and worth working on, because it can help the selection of new therapeutic targets. The therapies in early phase are more effective.
The aim of the present study was to investigate the changes of TNF- α , IL-1 β IL-10, IL-12 and IL-18 expressions during early phase (within 120 min) of endotoxic shock in macaques.
Totally 25 macaques (Macaca mulatta) of 5-8 years old, weighing 4.8-9.2 kg (6.17 ± 1.1 kg), were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. All animal studies were carried out in accordance with the institutional regulations concerning animal experimentation of the Ministry of Health of China. The animals were housed at 10-25 °C with light, around 8-10 h per day for, at least, a week before experiment. One of 25 macaques was used in preliminary experiment for the optimal dose of LPS at which hypotension and shock should be induced. The other 24 macaques were divided into two groups: 19 in LPS group and 5 in normal saline (NS) group.
LPS, derived from E. coli O127: B8 and prepared by phenol extraction, was purchased from Sigma Co. (Saint Louis, USA). ELISA kits for detecting human TNF- α , IL-1 β , IL-10 and IL-12 P40/ P70 were from Pharmingen (San Diego, USA). TriZol RNA extraction kit was from Gibco (Grand Island, USA). Moloney murine leukemia virus (M-MLV) reverse transcriptase, RNase inhibitor, PCR kit and PGEM-T vector were all from Promega (Shanghai, China). PCR product purification kit was from Boehringer Mannheim Co. (Mannheim, Germany). Plasmid DNA extraction kit was from Scientz Bio Co. (Shanghai, China).
In order to determine the mRNA expression levels of inflammatory cytokines by RT-PCR, some pairs of primers based on specific sequences published previously were used. These primers, synthesized in ShengGong Biotech Co. (Shanghai, China), are shown in Table 1.
| Cytokine | Primer | Sequence | Reference |
| Human TNF- α | upstream | TCA CAG GGC AAT GAT CCC AAA GTA GAC CTG C | [2,3] |
| downstream | ATG AGC ACT GAA AGC ATG ATC | ||
| Human IL-1 β | upstream | TTA GGA AGA CAC AAA TTG CAT GGT GAA | [2,3] |
| downstream | ATG GCA GAA GTA CCT GAG CTC | ||
| Human IL-18 | upstream | GCT TGA ATC TAA ATT ATC AGT C | [4] |
| downstream | GAA GAT TCA AAT TGC ATC TTA T | ||
| Human β -actin | upstream | TTC CAG CCT TCC TTC CTG G | [5] |
| downstream | TTG CGC TCA GGA GGA GCA AT |
In the pilot experiment, one macaque was used to find out optimal dose of LPS. Intravenous injection of LPS of 2.8 mg/kg resulted in reduction of mean arterial pressure (MAP) from 17.55 to 10.80 kPa. This dosage was applied to establish macaque model of endotoxic shock in this experiments. After being fasted overnight, macaques were anesthetized with ketamine (15 mg/kg, intramuscularly), then they were mechanically ventilated with a ventilator (SERVE900c, Siemens- Elama) through trachea intubator. Tidal volume was set at 12 mL/kg and the respiratory rate at 20 beat/min during experiment. A value of 5 cm H2O (1 cm H2O = 0.098 kPa) of positive end-expiratory pressure was applied to maintain end-expiratory lung volume. Anesthesia was maintained with repeated intravenous injection of sodium γ -hydroxybutyl acid ( 200 mg/kg·h ) and intramuscular injection of fentanylicitras ( a bolus of 6 μg/kg·30 min). A pulmonary artery catheter was placed in the jugular vein for detecting hemodynamic parameters by thermodilution (Siemens,). After percutaneous puncture, the catheter was introduced into the femoral artery and forwarded into the abdominal aorta for measuring blood pressure. Nineteen animals in the shock group were given a dose of 2.8 mg/kg LPS i.v., while 5 animals in the control group were given 1 mL/kg normal saline. Occurrences of endotoxic shock were confirmed by reduction of MAP by 30% and by other hemodynamic changes, such as the reduction of systemic vascular resistance index (SVRI) or left ventricular work index (LVWI), in LPS group. It took 60 min on average to establish shock after LPS injection. Venous blood of 18 animals in LPS group and 5 animals in NS control group were collected at 0, 60 and 120 min after LPS or NS injection for detection of inflammation-associated cytokines. Then, the macaques were sacrificed for detecting myocardial damages in another sub-study of this project. At the same time, a small portion of liver and spleen tissues were sliced and stored at -80 °C after being rapidly frozen in liquid nitrogen for mRNA assays.
Blood from the macaques, anticoagulated with EDTA-Na2, were centrifuged, at 1500 r/min × 10 min, to separate plasma from cells. The plasma were stored at -20 °C for cytokines detection. The sedimentary cells were resuspended with Hanks’ solution and the cell suspension was centrifuged with Ficoll-Hypaque lymphocyte separating medium, at 2 000 r/min × 20 min, to get peripheral blood mononuclear cells (PBMCs). After being rapidly frozen in liquid nitrogen, PBMCs from the macaques were stored at -80 °C for mRNA assays.
The levels of TNF-α , IL-1 β , IL-10 and IL-12P40/P70 in plasma were determined by double-antibodies sandwich ELISA using the kits. The assays were performed according to the kit protocol.
PBMCs of 106 and liver or spleen tissue of 0.1 g were used to extract total cytoplasmic RNA according to the TriZol method. The total RNA was converted to complementary DNA (cDNA) by a reverse transcription step with M-MLV reverse transcriptase, OligodT primer, RNase inhibitor and dNTPs. Using the cDNA, specific primers, SYBR-Green I and PCR kit, a series of real-time semi-quantitative PCR were performed with a DNA amplifier (PE5700) to determine levels of mRNA coding for TNF- α , IL-1 β and IL-18, as well as β -actin which was used in the assay as an internal control, in PBMCs, liver and spleen. cDNA samples were amplified for 40 cycles: denaturation at 94 °C for 30 s, annealing at 60 °C for 60 s and extension at 68 °C for 120 s. The ratio of fluorescence intensity of cytokine-specific product to that of the internal control product represents relative levels of cytokine mRNA expression. After PCR, the products were cloned into PGEM-T vector, which was then used to transform JM109 E. coli. The plasmid DNAs of positive clones were extracted for sequencing.
All results were expressed as mean ± SD. The significances of differences between LPS shock group and NS control group were evaluated by t-test. Testing standard was set at α = 0.05. P < 0.05 indicates a significant difference, and P < 0.01 indicates a remarkably significant difference.
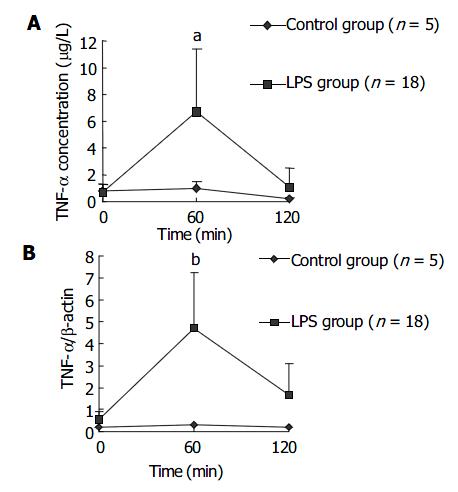
Plasma concentration of TNF-α in LPS group increased earlier and was significantly higher than that in NS group at 60 min after LPS administration, then decreased to a level similar to that in NS group. This result is shown in Figure 1A. The dynamics for TNF-α mRNA expression by PBMCs in LPS group was similar to that for plasma TNF-α concentration. The result is shown in Figure 1B.
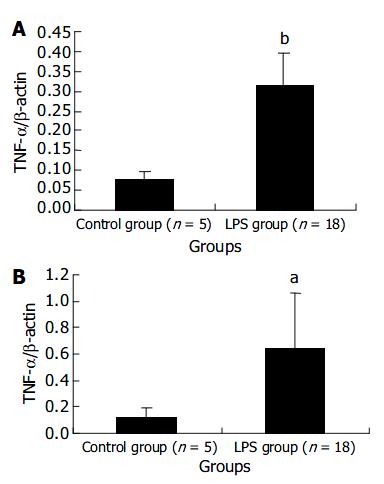
The expression levels of TNF-α mRNA in liver and spleen in LPS group were remarkably higher than those in NS control group at 120 min after LPS challenge. The results were presented in Figure 2.
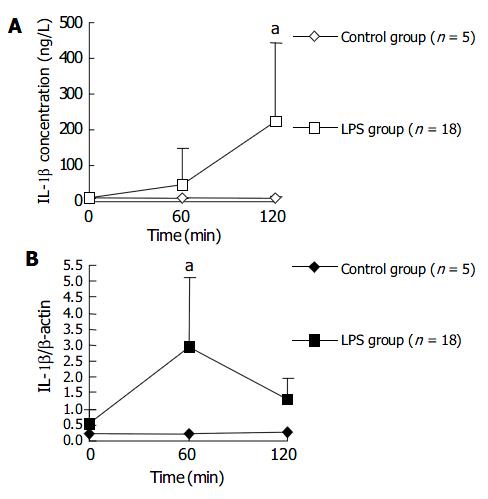
The plasma IL-1 β level in LPS group began to rise at 60 min after LPS challenge and was significantly higher at 120 min after LPS challenge, compared with that in NS control group. Whereas the IL-1 β transcription level in PBMCs rose earlier than plasma IL-1 β level. The transcription level of IL-1 β in PBMCs of LPS group was significantly higher than that in control group at 60 min after LPS injection, and then dropped to a level similar to that in control group after another 60 min. Figure 3 shows the changes of plasma level and transcription level of IL-1 β within 120 min after LPS injection.
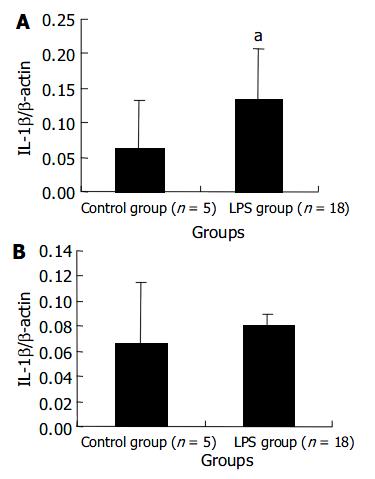
The transcript level of IL-1 β mRNA in the liver of LPS group remarkably increased at 120 min after LPS administration. But the level in the spleen did not show a significant increase. These results are shown in Figure 4.
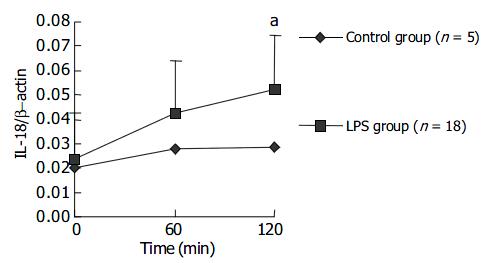
The expression level of IL-18 mRNA in PBMCs gradually increased with LPS challenge and a statistical significance could be found at 120 min after LPS challenge. The results are presented in Figure 5. Data in Figure 6 indicate at 120 min after LPS challenge, the expression levels of IL-18 mRNA in LPS group were significantly higher in liver cells, not in spleen cells, compared with those in control group after NS injection.
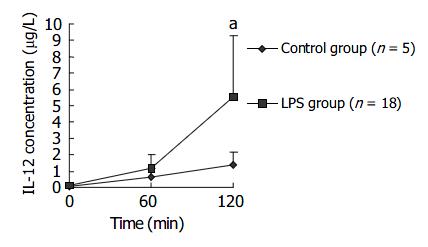
Sixty minutes after LPS challenge, plasma IL-12P40/P70 level of macaques in LPS group gradually rose and was significantly higher than that in control group at 120 min after LPS challenge. Data are supplied in Figure 7.
Immediately after LPS administration, plasma IL-10 level of macaques in LPS group increased slightly, and showed a statistically significant rise at 60 min after LPS injection, compared with control group. Thereafter the level gradually declined to normal level. The results are shown in Figure 8.
A series of endotoxic shock animal models have been established for studies. Macaque Mulatta belongs to primate animals. The physiological functions and anatomical features are similar among primates, including humans. Therefore, it is important to study primate model of endotoxic shock in order to understand septic shock in human. In the present study, an endotoxic shock model of macaque was established to study about changes of inflammation-associated cytokines during early phase of endotoxic shock.
In human volunteers, an intravenous injection with endotoxin bolus of 2-4 ng/kg caused pyrexia, cytokine release and mild decline of MAP[6,7]. In non-human primate, however, LPS sensitivities were quite different: chimpanzee shared the sensitivity of human to LPS, while baboons and rhesus macaques were insensitive to LPS like rodents[8,9]. Chimpanzee is too precious to be commonly used in research, baboon and macaque are used as primate model more commonly. The injection bolus of LPS of 10-20 mg/kg, or continuous infusion of LPS of 10 mg/(kg·h) was applied in several studies with rhesus macaques[10,11]. A bolus injection of LPS 3.0 mg/kg for Macaque mulatta model had been reported previously by Hajek[12]. In the present study, we successfully established an endotoxic shock model of Macaque mulatta, which was proved by the decline of MAP, SVRI and LVWI. The LPS dose we used was 2.8 mg/kg and consistent with the previous report in Macaque mulatta model[12].
Unfortunately, no standard and commercial ELISA kits for detection of macaque cytokines are available. Nevertheless, it is reasonable to try application of human cytokine kits to detect macaque cytokines, because there is a high homology in genome between human and primate animals such as macaque[2] and it was reported that IL-2 receptor on the surface of rhesus monkey cells reacted with antibody against human IL-2 receptor[13] and IL-1, IL-2 and TNF-α from monkey cells could bind to antibodies against human IL-1, IL-2 and TNF-α in ELISA[13,14]. In this experiment, we tried to use ELISA kits for detection human cytokines to determine the levels of TNF-α , IL-1 β , IL-12P40/P70 and IL-10 of Macaque mulatta and successfully obtained useful data.
Similarly, we used the primers based on human TNF-α , IL-1 β , IL-18 and β -actin genes to amplify the cDNA segments of macaque TNF-α , IL-1 β , IL-18 and β -actin by RT-PCR methods and successfully obtained the expected products such as 702-bp segment of TNF-α , 810-bp segment of IL-1 β , 341-bp segment of IL-18 and 225-bp segment of β -actin (Figure 9). The PCR products from one macaque were sequenced for homology comparison with human homologue. The results indicated that the identity of TNF-α or IL-18 gene segment between macaque and human was 97%, IL-1 β was 93% (Figure 10).
Semi-quantitative analysis of mRNA based on RT-PCR can be completed by using β -actin mRNA as an internal standard and by densitometry of product electrophoresis band scanned or product dot hybridized with specific probe. The relative index (RI) of mRNA is calculated using a formula as RI = density scan value of specific product/density scan value of internal control. Here, RI may represent the relative level of specific mRNA. In the present study, we also used β -actin mRNA as an internal control. In order to increase detection sensitivity, we used SYBR-Green I, a kind of fluorescence stain for ds-DNA, in the quantitative analysis. Moreover, a two step strategy of reverse transcription first and then PCR was applied to ensure the consistency of amplification efficiency between cytokine and control mRNA. On the other hand, a real-time PCR was performed to find the cycle number of exponential phase of target DNA. Because the cycle number of exponential phase of cellular target DNA is different from that of primer-dimer DNA, it is possible to exclude primer-dimer interference with target DNA quantitative analysis when the product of cellular target DNA is collected within its exponential phase. In our experiment, the cycle numbers of exponential phase of cellular target DNA were all less than 40, while that of primer-dimer was far more than 40. So the PCR was carried out for 40 cycles.
TNF-α is one of the most important inflammatory cytokines and has a lot of cellular origins such as monocyte-macrophages, lymphocytes and endotheliocytes. The present study demonstrated the level of plasma TNF-α peaked at 60 min after LPS injection and then gradually decreased. The change of mRNA expression level in PBMCs was nearly synchronous, which indicated PBMCs were one of main sources of plasma TNF-α . After LPS challenge, expression levels of TNF-α mRNA both in liver and in spleen increased, suggesting that liver and spleen were also the sources of plasma TNF-α . TNF-α releases firstly during infection or inflammatory reaction. In endotoxic shock models of rats, rabbits and monkeys, plasma TNF-α levels were reported to increase at 30 min after LPS injection and reach the peak about 60-120 min after LPS injection[15], which was in great agreement with our results. Kupffer cells in liver were an important source of TNF-α . It was reported in endotoxin-induced liver injury model of mice, there were two expression peaks of TNF-α mRNA in liver, one happened within 2 h after LPS injection and the other happened 5 h after LPS injection. The first peak, during which TNF-α was derived from LPS-activated kupffer cells, was not as high as the second one. The second peak was induced by other inflammatory cytokines such as INF-γ and IL-18, and was the main factor inducing liver injury[16]. In the present experiment, the increase of TNF-α mRNA in liver at 120 min after LPS injection should be the first peak. We found the expression level of TNF-α mRNA in spleen cells also increased after LPS injection. There are few studies on TNF-α production by spleen in endotoxic shock models, which needs more investigations.
IL-1 β , produced by activated monocyte-macrophages, is also an important inflammatory cytokine that is released early. Leturcq[17] reported that the peak of plasma IL-1 β level in endotoxic shock model of cynomolgus monkeys appeared at 2 h after LPS stimulation which was slightly later than that of plasma TNF-α . This is similar to our result. We observed that IL-1 β plasma level increased in 2 h after LPS injection, but later than TNF-α level did. The fact that expression level of IL-1 β mRNA in PBMCs increased significantly at 60 min after LPS injection and quite earlier than plasma level suggested that PBMCs were the main source of circulating IL-1 β . PBMCs synthesized and released IL-1 β firstly and IL-1 β accumulated in plasma sequentially. The liver cells were also one of IL-1 β sources, but spleen cells the not.
IL-18, produced mainly by kupffer cells and other macrophages, is another important inflammatory factor. IL-18 activates cytokine network of liver and inflammatory reaction. By inducing INF-γ , IL-18 causes the second peak of TNF-α and sequential increase of FasL which induces apoptosis of liver cells[16-18]. IL-18 was found to be expressed basically in fresh-separated human PBMCs and to increase significantly in 1 h after LPS stimulation[4]. In LPS-induced acute liver injury model in Propionibacterium acnes-primed INF-γ -deficient mice , IL-18 was found to show a basic expression in liver followed by a remarkably increased expression within 2 h after stimulation with Propionibacterium acnes and LPS[19]. In this study, we found in endotoxic shock model of macaques, IL-18 mRNA expression in PBMCs began to increase after LPS stimulation and showed a statistically significant increase 2 h later. We also demonstrated a significant increase of IL-18 transcription in liver cells after LPS stimulation. These discoveries in macaque models were not reported here before.
IL-12, produced mainly by macrophages, kupffer cells and dendritic cells, activates neutrophilic granulocytes and promotes release of inflammatory cytokines such as INF-γ , TNF-αetc.[20,21], while inhibits the release of anti-inflammation cytokines such as IL-10[22]. So, IL-12 is also closely related to inflammatory responses. Reports about the change of IL-12 expression in endotoxic shock was quite few except for Tsuji’s work, in which plasma IL-12 level of acute liver injury model mice was reported to reach a peak within 3 h after LPS stimulation, following the first peak of TNF-α [19]. In Trinchieri’s opinion, the observation of dynamics of inflammatory cytokines in above model mice suggested that the order of activation for these cytokines was: the first peak of TNF-α , IL-12, IL-18, INF-γ , the second peak of TNF-α and FasL[23]. Our observation indicated in macaque mulatta, the plasma IL-12 concentration significantly increased in 120 min after LPS stimulation. To our knowledge, there were few reports about IL-12 dynamics in endotoxic shock primate model. Our results in macaque were compatible with Trinchieri’s description.
IL-10, originated from Th1, Tr (regulator) or Th3 cells and B lymphocytes as well as monocyte-macrophages, is an anti-inflammation cytokine, inhibiting the release of inflammation cytokines such as TNF-α , IL-1 β , IL-6, IL-8, IL-12 and IL-18[24,25]. It is one of the cytokines produced very early after LPS injection in mice model, like TNF-α . Dynamics curves showed two peaks of plasma IL-10 in mice model: plasma IL-10 level reached the first peak in 1-5 h after LPS injection and then declined; the second peak appeared in 8-12 h after LPS injection. Plasma IL-10 originated from kupffer cells in liver during the first peak, while it originated from peripheral blood lymphocytes during the second peak[26]. The amount of IL-10 in the first peak during early phase of endotoxic shock was not enough to suppress the increases of inflammatory cytokines and hence effectively stop inflammation. So it is necessary to use exogenous recombinant IL-10 as a therapeutics in early phase of endotoxic shock to interrupt the chain release of inflammatory cytokines and to stop shock development[27]. In the present study, we observed IL-10 level in shock model of macaques increased slightly, and significantly in 60 min after LPS injection and formed a smaller peak, and about 60 min later it returned nearly to the value of control group. Our result in macaque model was quite consistent with that in mouse model. The rise of IL-10 level we observed in early phase of endotoxic shock in macaque models should correspond to the first peak.
It is necessary to mention Kupffer cells for understanding the dynamics of inflammatory cytokine release induced by LPS. LPS is the main activator of Kupffer cells, while Kupffer cells are the major source of inflammatory cytokines, such as TNF-α , IL-12 and IL-18, which are produced in response to LPS. TNF-α is the key factor in systemic inflammation (for example, endotoxic shock and septic syndrome), and it is Kupffer cells that are the major source of TNF-α in the liver. So Kupffer cells play an important role in systemic inflammatory reaction caused by Gram-negative bacteria. On the other hand, Kupffer cells are the main scavengers of LPS and also play an important role in removing circulatory LPS and relieving the stimulation and injury caused by LPS[28].
Edited by Zhu LH Proofread by Chen WW and Xu FM
| 1. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961-965. [PubMed] |
| 2. | Villinger F, Brar SS, Mayne A, Chikkala N, Ansari AA. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946-3954. [PubMed] |
| 3. | Benveniste O, Vaslin B, Villinger F, Le Grand R, Ansari AA, Dormont D. Cytokine mRNA levels in unmanipulated (ex vivo) and in vitro stimulated monkey PBMCs using a semi-quantitative RT-PCR and high sensitivity fluorescence-based detection strategy. Cytokine. 1996;8:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999;96:2256-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 5. | Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466-472. [PubMed] |
| 6. | Pernerstorfer T, Schmid R, Bieglmayer C, Eichler HG, Kapiotis S, Jilma B. Acetaminophen has greater antipyretic efficacy than aspirin in endotoxemia: a randomized, double-blind, placebo-controlled trial. Clin Pharmacol Ther. 1999;66:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Boujoukos AJ, Martich GD, Supinski E, Suffredini AF. Compartmentalization of the acute cytokine response in humans after intravenous endotoxin administration. J Appl Physiol (1985). 1993;74:3027-3033. [PubMed] |
| 8. | Redl H, Bahrami S, Schlag G, Traber DL. Clinical detection of LPS and animal models of endotoxemia. Immunobiology. 1993;187:330-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Harper PL, Taylor FB, DeLa Cadena RA, Courtney M, Colman RW, Carrell RW. Recombinant antitrypsin Pittsburgh undergoes proteolytic cleavage during E. coli sepsis and fails to prevent the associated coagulopathy in a primate model. Thromb Haemost. 1998;80:816-821. [PubMed] |
| 10. | Richman AV, Okulski EG, Balis JU. New Concepts in the pathogenesis of acute tubular necrosis associated with sepsis. Ann Clin Lab Sci. 1981;11:211-219. [PubMed] |
| 11. | Premaratne S, May ML, Nakasone CK, McNamara JJ. Pharmacokinetics of endotoxin in a rhesus macaque septic shock model. J Surg Res. 1995;59:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Hájek M, Trcka V, Vanĕcek M, Helfert I, Misák J. A model of experimental endotoxin shock in monkeys and its therapeutic control. Z Exp Chir. 1978;11:317-321. [PubMed] |
| 13. | Schmitt DA, Sonnenfeld G, Husson D, Tkaczuk J, André E, Schaffar L. In vitro interleukin-1 and 2 production and interleukin 2 receptor expression in the rhesus monkey. Life Sci. 1996;59:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Verdier F, Aujoulat M, Condevaux F, Descotes J. Determination of lymphocyte subsets and cytokine levels in cynomolgus monkeys. Toxicology. 1995;105:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Carvalho GL, Wakabayashi G, Shimazu M, Karahashi T, Yoshida M, Yamamoto S, Matsushima K, Mukaida N, Clark BD, Takabayashi T. Anti-interleukin-8 monoclonal antibody reduces free radical production and improves hemodynamics and survival rate in endotoxic shock in rabbits. Surgery. 1997;122:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Tsutsui H, Matsui K, Kawada N, Hyodo Y, Hayashi N, Okamura H, Higashino K, Nakanishi K. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961-3967. [PubMed] |
| 17. | Leturcq DJ, Moriarty AM, Talbott G, Winn RK, Martin TR, Ulevitch RJ. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 436] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Tsuji H, Mukaida N, Harada A, Kaneko S, Matsushita E, Nakanuma Y, Tsutsui H, Okamura H, Nakanishi K, Tagawa Y. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-gamma-deficient mice by a concomitant reduction of TNF-alpha, IL-12, and IL-18 production. J Immunol. 1999;162:1049-1055. [PubMed] |
| 20. | Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1757] [Cited by in RCA: 1792] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 21. | Carson WE, Yu H, Dierksheide J, Pfeffer K, Bouchard P, Clark R, Durbin J, Baldwin AS, Peschon J, Johnson PR. A fatal cytokine-induced systemic inflammatory response reveals a critical role for NK cells. J Immunol. 1999;162:4943-4951. [PubMed] |
| 22. | Marshall JD, Secrist H, DeKruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4 + T lymphocytes. J Immunol. 1995;155:111-117. [PubMed] |
| 23. | Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Németh ZH, Haskó G, Vizi ES. Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-alpha, MIP-1alpha, IL-12, and nitric oxide production and protects from the lethal effect of endotoxin. Shock. 1998;10:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Marshall JD, Aste-Amézaga M, Chehimi SS, Murphy M, Olsen H, Trinchieri G. Regulation of human IL-18 mRNA expression. Clin Immunol. 1999;90:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Barsig J, Küsters S, Vogt K, Volk HD, Tiegs G, Wendel A. Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor-alpha. Eur J Immunol. 1995;25:2888-2893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 583] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 28. | Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000;20:1860-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |