Published online Oct 15, 2004. doi: 10.3748/wjg.v10.i20.2979
Revised: January 21, 2004
Accepted: February 26, 2004
Published online: October 15, 2004
AIM: To design and construct an exogenous multiple epitope of helper T lymphocytes (HTL), and to evaluate its effect on anti-HBs response through DNA immunization.
METHODS: Artificial HTL epitope, PADRE and four other HTL epitopes from different proteins were linked together using splicing by overlap extension to generate exogenous multiple epitopes of HTL, MTE5. pcMTE5 and pcHB were generated by cloning MTE5 and fragments of HBV pre-S2/S gene into mammalian expression plasmid pcDNA3. Four chimeric plasmids were constructed by cloning MTE5 into the region of pre-S2 gene (Bam HI), 5’ terminal of S gene (HincII, Xba I) and 3’ terminal of S gene (Acc I) of pcHB respectively. BALB/c mice were used in DNA immunization of the recombinant plasmids. Anti-HBs was detected using Abbott IMx AUSAB test kits.
RESULTS: The sequences of MTE5 and the 6 constructs of recombinant plasmids were confirmed to be correct by DNA sequencing. The anti-HBs response of the co-inoculation of pcHB and pcMTE5 was much higher than that of the inoculation of pcHB only (136.7 ± 69.1 mIU/mL vs 27.6 ± 17.3 mIU/mL, P < 0.01, t = -6.56). Among the 4 chimeric plasmids, only the plasmid in which MTE5 was inserted into the pre-S2 region had good anti-HBs response (57.54 ± 7.68 mIU/mL), and had no significant difference compared with those of pcHB and the co-inoculation of pcHB and pcMTE5.
CONCLUSION: Exogenous multiple epitopes of HTL had immune enhancement when they were co-inoculated with pre-S2/S gene or inoculated in the chimeric form at a proper site of pre-S2/S gene of HBV. It might suggest that it was possible to improve hepatitis B vaccine using exogenous multiple epitopes of HTL. The antibody responses were very low using DNA immunization in the study. Thus, the immune enhancement effect of exogenous multiple epitopes of HTL has to be confirmed and the effect on overcoming the drawback of the polymorphism of HLA II antigens should also be evaluated after these chimeric plasmids are expressed in mammalian cell lines.
- Citation: Gao WJ, Peng XM, Xie DY, Xie QF, Gao ZL, Yao JL. Construction of exogenous multiple epitopes of helper T lymphocytes and DNA immunization of its chimeric plasmid with HBV pre-S2/S gene. World J Gastroenterol 2004; 10(20): 2979-2983
- URL: https://www.wjgnet.com/1007-9327/full/v10/i20/2979.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i20.2979
There are more than 300 millions of HBsAg carriers all over the world. Most of them have active infection of hepatitis B virus. Chronic hepatitis B virus infection may cause significant incidence of chronic hepatitis, cirrhosis, and hepatocellular carcinoma[1,2]. Hepatitis B vaccine inoculation is an important measure for prevention of HBV infection. Considerable variability exists, however, in response to hepatitis B vaccines, and 5-10% of healthy young adults demonstrate no or inadequate responses following a standard vaccination schedule[3-8]. The frequency of non- and low-responders is up to 40-50% in vaccined subjects with depressed immune responses, such as patients on maintenance hemodialysis. Only some of the non- or low-responders to hepatitis B vaccine are responsive to the novel recombinant triple antigen hepatitis B vaccine (pre-S1/S2 and HBsAg)[9]. These non- or low-responders usually carry specific type of class II human leukocyte antigens (HLA II antigens), such as DRB1*3, DRB1*7, DQB1*20 or DPB1*1101[10-13]. The antigen presentation is blocked because these HLA II antigen molecules on the surface of helper T lymphocytes (HTL) cannot combine efficiently with the epitope of HTL on the vaccine protein, hepatitis B surface antigen (HBsAg)[14,15]. Adding exogenous multiple epitopes of HTL, which can combine with much more types of HLA II antigens to hepatitis B vaccine may be a good way to solve this problem. Conducting HTL epitopes from tetanus toxoids (aa73-99, aa830-845) and artificial epitopes of PADRE into HBsAg can enhance the immunogenicity of HbsAg[16,17]. Adding exogenous HTL epitopes to HBsAg can let natural non-responder mice respond to HbsAg[18]. These data suggest that it is possible to use exogenous HTL epitopes to improve hepatitis B vaccine. For these reasons, an exogenous multiple epitope of HTL, MTE5, consisting of five HTL epitopes from different origins was designed and constructed. Its effect on anti-HBs response was evaluated through DNA immunization after it was inserted in different loci of HBV pre-S2/S gene.
pTZ19U-HBV containing double copies of HBV DNA (adw) was a gift from professor Zhi-Min Huang, Sun Yat-Sen University. T4 DNA ligase and pfu DNA polymerase were purchased form Promega Company (USA). DNA gel extraction kits and plasmid isolation kits were purchased from Qiagen Company (German). Polyclonal antibodies of anti-HBs and LSAB test kit for immunohistochemistry were purchased form DAKO Company (USA). Primers and oligonucleotide fragments shown in Table 1 were synthesized in Bioasia Biological Engineering Company (Shanghai, China).
| Sequences (5’→3’) | |
| F1 | ATGGCTAAAA CCATCGCCTA TGATGAAGAA GCTCGTCGTG GTCTGGAACG TGGTCTGAAT GCCTCCGAT |
| F2 | GTCCAGGCAG CAACGAATTT AGCTCCCTGC AGCCAGTGTC CAGGCAGAAC ATCGGAGGCA TTCAGACCA |
| F3 | AATTCGTTGC TGCCTGGACC CTGAAAGCTG CCGCTGGAAG ACACGTTGTT ATCGATAAGA GCTTCGGAA |
| F4 | TTCGGTGATT CCGATAAACT TGGAATTAGC TTTGATGTAC TGCTGAGGGC TTCCAAGGCT CTTATCGAT M ` T E - |
| MTE-P1 | TCGGATCCCA TGGCTAAAAC CATCGCC |
| MTE-P2 | GCTCTAGACT TTCGGTGATT CCGATAA |
| HBV-P1 | CCTAAGCTTA TGCAGTGGAA CTCCACT |
| HBV-P2 | TGGAATTCCT TAAATGTATA CCCAGAG |
| Bam-1 | TCGGATCCCA TGGCTAAAAC CATCGCC |
| Bam-2 | GCGGATCCCT TTCGGTGATT CCGATAA |
| Hinc-1 | TCGTTGACAA TGGCTAAAAC CATCGCC |
| Hinc-2 | GCGTTGACAA TTCGGTGATT CCGATAA |
| Xba-1 | TCTCTAGACA TGGCTAAAAC CATCGCC |
| Xba-2 | GCTCTAGACT TTCGGTGATT CCGATAA |
| Acc-1 | TCGTATACAT GGCTAAAACC ATCGCCT |
| Acc-2 | CGCGTATACT TCGGTGATTC CGATAAA |
Eight to 12 week-old female inbred BALB/c mice were obtained from Guangzhou Traditional Chinese Medicine University.
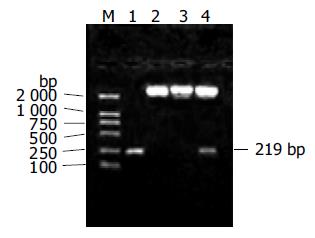
MTE5 splicing and cloning MTE5 was designed to consist of HTL epitopes from heat shock protein 65 of mycobacterium tuberculosis (aa1-20), E2 polyprotein of rubella virus (aa54-65), artificial epitope (PADRE), heat shock protein 60 of chlamydia trachomatis (aa35-48) and tetanus toxoid (aa830-843). These epitopes were translated into a 219-bp fragment of DNA sequence. Then, it was synthesized in four oligonucleotide fragments, which were linked together at last using splicing by overlap extension[19,20]. The oligonucleotide fragments F1 and F2, F3 and F4 were spliced by PCR respectively at first. Their purified products were spliced together again to generate MTE5. The construct of pUMTE5 was generated by cloning MTE5 into plasmid pUC18 at the site between restriction endonucleases Bam HI and Xba I. pcMTE5 then was obtained by sub-cloning MTE5 from pUMTE5 into mammalian expression plasmid, pcDNA3. The sequence of MTE5 in pcMTE5 was confirmed using automatic DNA sequencing in Bioasia Biological Engineering Company.
HBV pre-S2/S gene cloning Pre-S2/S fragment of HBV was obtained using pTZ19U-HBV as template, and HBV-P1 and HBV-P2 as primers. The purified product was then cloned into pUC18 at the site between the restriction endonucleases Hind III and Eco RI to generate recombinant plasmid pUHB. pcHB was constructed by sub-cloning the fragment of HBV pre-S2/S gene into pcDNA3. pcHB was denominated after its sequence was confirmed by automatic DNA sequencing.
Construction of chimeric plasmids of MTE5 and HBV pre-S2/S gene MTE5 was designed to insert into the region of pre-S2 gene (Bam HI), 5’ terminal of S gene (Hinc II, Xba I) and 3’ terminal of S gene (Acc I) respectively. All the inserting sites were far from the α determinant to avoid deceasing the antigenicity of HBsAg. In order to obtain these chimeric plasmids, four fragments of MTE5 with different types of restriction endonuclease site in its two terminals were generated through PCR with different primers at first. Then four chimeric plasmids, pcHB-MTEB, pcHB-MTEH, pcHB-MTEX and pcHB-MTEA were constructed by cloning MTE5 into pUHB at first since pcDNA3 had some similar restriction endonuclease sites of its own, and then by sub-cloning the chimeric fragment of MTE5 and pre-S2/S into pcDNA3. The sequences of these recombinant plasmids were confirmed by automatic DNA sequencing.
Large-scale DNA preparation, DNA immunization Large-scale plasmid DNA of recombinant plasmids was prepared using Qiagen’s Max-Prep kits. Plasmid DNA of pcDNA3 was used as control. Plasmid DNA was adjusted to 1 µg/mL in normal saline. Seventy BLBA/c mice were randomly divided into 8 groups. Every mouse was injected a total 100 µL of plasmid DNA which was distributed over five different sites into the anterior tibialis muscle 5 d after the injection of an equal volume of 2 g/L Bupivacaine. Boost injection was carried out every 3 wk for 3 times with an equal amount of plasmid DNA. Four weeks after the last boost injection, all mice were put to death for serum.
Detecting anti-HBs in serum Anti-HBs in serum was detected using AUSAB kits (Abbott Laboratory, USA). The tests were carried out following the manufacturer’s instructions.
Pathological examination and HBsAg detection in muscle cell Pathological examination and HBsAg detection using immunohistochemistry in the mouse muscle of inoculation site from all groups were carried out after the samples were routinely embedded by paraffin in our laboratory. The muscles from the opposite leg were used as negative controls.
Statistical analysis For anti-HBs level, geometric mean titer (GMT) for each group was calculated at first. Then Student-Newman-Keuls-q was used for statistical analysis. For positive rate, Fisher’s exact probability analysis was used. SPSS 10.0 for Windows was used for all statistical analyses.
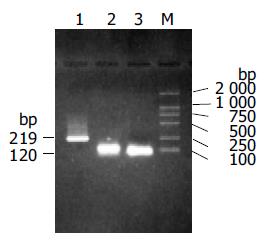
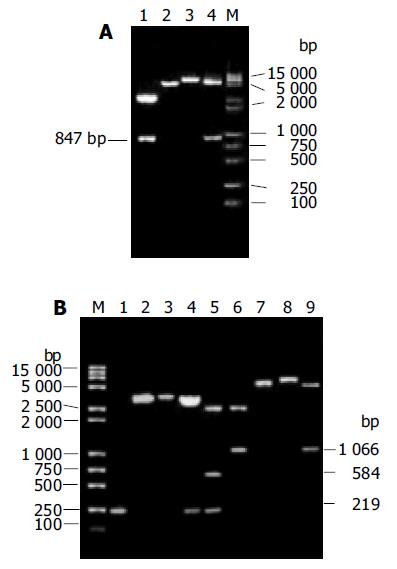
The theoretical products of the first and second round splicing were 120 bp and 219 bp, respectively. The actual products shown in Figure 1 were same as the design. Restriction fragment length polymorphism (RFLP) analysis was used to identify the positive clones after the 219-bp fragment was inserted into pUC18 to generate recombinant plasmid pUMTE5, which is shown in Figure 2. Two correct clones were selected by DNA sequencing among 13 positive clones of pUMTE5. The rest clones usually carried deletions or mismatches in the region longer than 50 bp of the synthesized oligonucleotide fragments, which implied that the synthesized DNA oligonucleotide fragment longer than 50 bp would not be reliable. The DNA sequence and amino acid sequence of MTE5 are shown in Figure 3. MTE5 was successfully cloned into pcDNA3 to generate pcMTE5 at last.
The theoretical base pair number of PCR products of HBV pre-S2/S fragment was about 847 bp. It was cloned into pUC18 to generate recombinant plasmid pUHB. Then, the fragment of pre-S2/S was sub-cloned into pcDNA3 to generate recombinant plasmid pcHB. RFLP analysis was used to identify the positive clones of pcHB, which is shown in Figure 4A. After its sequence was confirmed by DNA sequencing, the recombinant plasmid was denominated as pcHB.
Four fragments of MTE5 with different types of restriction endonuclease site in its two terminals were successfully obtained by PCR. They were cloned into pUHB to generate their recombinant plasmids at first. The chimeric fragments of MTE5 and pre-S2/S were sub-cloned into pcDNA3 to generate their mammalian expression plasmids at last. RFLP analysis was used to identify positive clones. The results of RFLP analysis of the recombinant plasmid pcHB-MTEX with MTE5 inserted in site of Xba I is shown in Figure 4B as an example. The sequence of all chimeric plasmids was confirmed to be correct by DNA sequencing.
All mice were alive after the inoculation schedule was finished. The anti-HBs levels are shown in Table 2. The anti-HBs level was negative in groups of normal saline and plasmid pcDNA3. The anti-HBs response to co-inoculation of pcHB and pcMTE5 was much higher than that to inoculation of pcHB only (136.7 ± 69.1 mIU/mL vs 27.6 ± 17.3 mIU/mL, the positive rate was 8/10 vs 6/10, P < 0.01, t = -6.56). Among the 4 chimeric plasmids, only pcHB-MTE-B with MTE5 inserted into pre-S2 region had a good anti-HBs response, which was 57.54 ± 7.68 mIU/mL, and slightly higher than that of pcHB, and lower than that of the co-inoculation of pcHB and pcMTE5. However, the difference was not statistically significant (P > 0.05).
Compared with the control groups and the sample from the opposite leg, all experimental groups had obvious inflammation cell filtration in the inoculation sites and HBsAg expression in muscle cells with disassembling and rupture. HBsAg was prominently expressed in cytoplasm of cells. Some cells had membrane expressions simultaneously. The positive signal of HBsAg was usually expressed in cytoplasm, and inflammation was more obvious in non- or low-anti-HBs response groups of chimeric plasmids.
The simple way for a given protein to obtain exogenous multiple epitopes was to link them to a higher molecular weight protein. The immune responses were not always good enough because of the interfering effects of immune responses of the carrying protein[21]. The spliced exogenous multiple epitopes of HTL used in this study, however, might not have immune responses of its own, and were thus preferable. The exogenous multiple epitopes of HTL, MTE5, constructed in this study consisted of 2 universal HTL epitopes and 3 unique HTL epitopes. Two universal epitopes of tetanus toxoid (aa830-843) and artificial epitope PADRE were both effective for 95% individuals of the population[22-26]. They might cover more individuals when used together. Three unique epitopes from Mycobacterium tuberculosis (aa1-20), E2 polyprotein of rubella virus (aa54-65) and heat shock protein 60 of Chlamydia trachomatis (aa35-48) were selected for HLA-DRB1*3, HLA-DRB1*7 and HLA-DR4 respectively[27-29]. They could improve the anti-HBs responses of hepatitis B vaccine in individuals who carried these types of HLA II antigens. Thus, the overall response rate of HB vaccine in population could rise dramatically. During the course of splicing, many deletions or mismatches were found in the spliced sequences, especially in the region of synthesized oligonucleotide fragments longer than 50 bp. It implied that synthesized DNA oligonucleotide fragments shorter than 50 bp would be preferable.
A satisfactory ant-HBs response was obtained when MTE5 and pre-S2/S were co-inoculated through DNA immunization, suggesting that MTE5 had immune enhancement. Among the 4 chimeric plasmids, only the recombinant plasmid with MTE5 inserted into the region of pre-S2 had a detectable anti-HBs response, suggesting that the insertion location of MTE5 was critical for anti-HBs response. The insertion was far from the a antigen determinant of HBsAg. Thus, the non-response cause of the rest chimeric plasmids was not clear. HBsAg could be detected in muscle cells at the inoculation site, suggesting that the construction of recombinant plasmids was successful. HBsAg positive signals were mainly located in cytoplasm, suggesting that the poor anti-HBs response might result in failure to secret the large particles like HBsAg[30-32].
The chimeric plasmid of pcHB-MTEB had an equal or better anti-HBs response as comparing with that of pcHB, and might be a good candidate for new hepatitis B vaccine in further research. DNA immunization is a simple and quick way to evaluate recombinant plasmids. The response rate of recombinant plasmids, however, was very low in this study. A considerable portion of mice did not develop anti-HBs at all. Its reason is not clear. It might be the nature of DNA immunization because this phenomenon also occurred in other researches[31]. The exact value should be evaluated after purified recombinant protein is obtained through mammalian expression. The effect on overcoming the drawback of the polymorphism of HLA II antigens should also be evaluated in the future.
Edited by Wang XL and Chen WW Proofread by Xu FM
| 1. | Liaw YF. Management of patients with chronic hepatitis B. J Gastroenterol Hepatol. 2002;17:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 374] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 3. | Das K, Gupta RK, Kumar V, Kar P. Immunogenicity and reactogenicity of a recombinant hepatitis B vaccine in subjects over age of forty years and response of a booster dose among nonresponders. World J Gastroenterol. 2003;9:1132-1134. [PubMed] |
| 4. | Kubba AK, Taylor P, Graneek B, Strobel S. Non-responders to hepatitis B vaccination: a review. Commun Dis Public Health. 2003;6:106-112. [PubMed] |
| 5. | van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Wang J, Zhu Q, Zhang X. Effect of delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J (Engl). 2002;115:1510-1512. [PubMed] |
| 7. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 610] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 8. | Tsebe KV, Burnett RJ, Hlungwani NP, Sibara MM, Venter PA, Mphahlele MJ. The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine. 2001;19:3919-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Page M, Jones CD, Bailey C. A novel, recombinant triple antigen hepatitis B vaccine (Hepacare). Intervirology. 2001;44:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Thio CL, Thomas DL, Karacki P, Gao X, Marti D, Kaslow RA, Goedert JJ, Hilgartner M, Strathdee SA, Duggal P. Comprehensive analysis of class I and class II HLA antigens and chronic hepatitis B virus infection. J Virol. 2003;77:12083-12087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Höhler T, Reuss E, Evers N, Dietrich E, Rittner C, Freitag CM, Vollmar J, Schneider PM, Fimmers R. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet. 2002;360:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Desombere I, Willems A, Leroux-Roels G. Response to hepatitis B vaccine: multiple HLA genes are involved. Tissue Antigens. 1998;51:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Watanabe H, Matsushita S, Kamikawaji N, Hirayama K, Okumura M, Sasazuki T. Immune suppression gene on HLA-Bw54-DR4-DRw53 haplotype controls nonresponsiveness in humans to hepatitis B surface antigen via CD8+ suppressor T cells. Hum Immunol. 1988;22:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Höhler T, Meyer CU, Notghi A, Stradmann-Bellinghausen B, Schneider PM, Starke R, Zepp F, Sänger R, Clemens R, Meyer zum Büschenfelde KH. The influence of major histocompatibility complex class II genes and T-cell Vbeta repertoire on response to immunization with HBsAg. Hum Immunol. 1998;59:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mineta M, Tanimura M, Tana T, Yssel H, Kashiwagi S, Sasazuki T. Contribution of HLA class I and class II alleles to the regulation of antibody production to hepatitis B surface antigen in humans. Int Immunol. 1996;8:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Chengalvala MV, Bhat RA, Bhat BM, Vernon SK, Lubeck MD. Enhanced immunogenicity of hepatitis B surface antigen by insertion of a helper T cell epitope from tetanus toxoid. Vaccine. 1999;17:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Peng XM, Xie DY, Gu L, Huang YS, Gao ZL, Yao JL. [Effect of exogenous epitopes of helper T lymphocyte on humoral immunity of HBV S gene DNA immunity]. Zhonghua Yixue Zazhi. 2003;83:232-236. [PubMed] |
| 18. | Hervás-Stubbs S, Berasain C, Golvano JJ, Lasarte JJ, Prieto I, Sarobe P, Prieto J, Borrás-Cuesta F. Overcoming class II-linked non-responsiveness to hepatitis B vaccine. Vaccine. 1994;12:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2407] [Cited by in RCA: 2517] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 20. | An LL, Whitton JL. A multivalent minigene vaccine, containing B-cell, cytotoxic T-lymphocyte, and Th epitopes from several microbes, induces appropriate responses in vivo and confers protection against more than one pathogen. J Virol. 1997;71:2292-2302. [PubMed] |
| 21. | Bârzu S, Arondel J, Guillot S, Sansonetti PJ, Phalipon A. Immunogenicity of IpaC-hybrid proteins expressed in the Shigella flexneri 2a vaccine candidate SC602. Infect Immun. 1998;66:77-82. [PubMed] |
| 22. | Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 564] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Boitel B, Blank U, Mège D, Corradin G, Sidney J, Sette A, Acuto O. Strong similarities in antigen fine specificity among DRB1* 1302-restricted tetanus toxin tt830-843-specific TCRs in spite of highly heterogeneous CDR3. J Immunol. 1995;154:3245-3255. [PubMed] |
| 24. | Valmori D, Sabbatini A, Lanzavecchia A, Corradin G, Matricardi PM. Functional analysis of two tetanus toxin universal T cell epitopes in their interaction with DR1101 and DR1104 alleles. J Immunol. 1994;152:2921-2929. [PubMed] |
| 25. | Franke ED, Hoffman SL, Sacci JB, Wang R, Charoenvit Y, Appella E, Chesnut R, Alexander J, Del Guercio MF, Sette A. Pan DR binding sequence provides T-cell help for induction of protective antibodies against Plasmodium yoelii sporozoites. Vaccine. 1999;17:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 428] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 27. | Young SP, Epstein E, Potter V. Determinant capture by MHC class II DR3 during processing of mycobacteria leprae 65kD heat shock protein by human B cells. Hum Immunol. 1998;59:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 28. | Ou D, Chong P, Choi Y, McVeigh P, Jefferies WA, Koloitis G, Tingle AJ, Gillam S. Identification of T-cell epitopes on E2 protein of rubella virus, as recognized by human T-cell lines and clones. J Virol. 1992;66:6788-6793. [PubMed] |
| 29. | Deane KH, Jecock RM, Pearce JH, Gaston JS. Identification and characterization of a DR4-restricted T cell epitope within chlamydia heat shock protein 60. Clin Exp Immunol. 1997;109:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Davis HL, Michel ML, Whalen RG. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum Mol Genet. 1993;2:1847-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 279] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Geissler M, Tokushige K, Chante CC, Zurawski VR, Wands JR. Cellular and humoral immune response to hepatitis B virus structural proteins in mice after DNA-based immunization. Gastroenterology. 1997;112:1307-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Geissler M, Schirmbeck R, Reimann J, Blum HE, Wands JR. Cytokine and hepatitis B virus DNA co-immunizations enhance cellular and humoral immune responses to the middle but not to the large hepatitis B virus surface antigen in mice. Hepatology. 1998;28:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |