Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2769
Revised: January 23, 2004
Accepted: February 21, 2004
Published online: September 15, 2004
AIM: To highlight the intestinal perforation (IP), an uncommon and catastrophic complication after combined liver-kidney transplantation.
METHODS: Combined liver-kidney transplantation (LKTx) with left kidney excision and a cyst fenestration procedure on the right kidney were performed on a case of 46-year-old female with congenital polycystic disease (CPCD).
RESULTS: Two sites of IP were noted 40-50 cm proximal to ileocecal area during emergent laparotomy 10 d postoperatively. Despite aggressive surgical and medical management, disease progressed toward a fatal outcome due to sepsis and multiple organ failure 11 d later.
CONCLUSION: Long duration of operation without venovenous bypass, overdose of steroid together with postoperative volume excess may all contribute to the risk of idiopathic multiple IPs. Microbiology and pathology inspections suggested that the infected cyst of the fenestrated kidney might be one reason for the fatal intra-peritoneal infection. Thus for the CPCD patients who seem to be very susceptible to infectious complications, any sign of suspected renal-infection found before or during LKTx is indication for the excision of original kidney. And the intensity of immunosuppression therapy should be controlled cautiously.
- Citation: Peng T, Peng MH, Li LQ, Deng YL, Yang DH, Lu BY, Chen XG, Guo Y, Xiao KY, Chen B, Zhong Q, Wei MY. Intestinal perforation after combined liver-kidney transplantation for a case of congenital polycystic disease. World J Gastroenterol 2004; 10(18): 2769-2771
- URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2769
Intestinal perforation (IP) is a catastrophic complication after liver or kidney transplantation and well documented in literatures separately[1,2]. Herein we report a case of IP after combined liver-kidney transplantation (LKTx).
The recipient was a 46-year-old female who had a history of 6-year aggravating abdominal girth and pain, with symptoms of gross hematuria, intermittent fever and anorexia in the recent one and a half year. The above symptoms severely affected her daily life as well as her profession. She had obvious abdominal protuberance with a liver palpable 5 cm below the right costal margin, the lower edge of left kidney aligned with the navel level and the right kidney 3 cm lower. Percussion tenderness was found in bilateral areas of kidney. Her liver function was Child B. Her serum creatinine was 132 μmol/L and the glomerular filtration rate was 19.3 mL/min. In urine sediment Gram-negative bacillus was found (1/hpf). ABO typing was “O”. HLA typing results were: A3, 26 (10); B39 (16), 54 (22); Bw6; DR4, 8; DRw53; DQw7 (3), 8 (3). Preoperative diagnosis was congenital polycystic disease (CPCD) with chronic renal failure and urinary tract infection.
Allografts came from an “O” type, 23-year-old male cadaver. HLA typing results were: A203 (2), 1102 (11); B27, 38 (16); Bw4; DR15 (2), 16 (2); DRw51; DQw5 (1), 6 (1); DTT-PRA negative; lymphocytotoxic crossmatch negative. Liver and kidney were harvested and preserved routinely.
Bacillus in urine sediment and culture turned negative after therapy whereas WBC counting was 4.1 × 109/L (neutrophils 80.5%) before operation. Left kidney excision + right kidney fenestration + combined liver-kidney transplantation were performed on June 23, 2000. About 1000 mL ascites was found in laparotomy. The fluid in the cysts of right kidney and liver was clear, in contrast to those muddy yellow-white liquids in the cysts of left kidney. Culture of the fluid from left cysts showed Gram-negative bacilli. The recipient hepatectomy and graft liver implant (Piggyback) were done routinely with modification (the hepatic artery and portal vein anastomosis was performed subsequently and revascularized simultaneously). No venovenous bypass was done. The left kidney resection, right kidney depression and graft kidney implantation (in right iliac fossa) were performed in the usual manner. Totally 650 mL urine was collected from recipient’s kidneys but no urine drainage was found from the implanted kidney. The whole operation lasted 18 h and 47 min (anhepatic period 2 h 7 min; warm ischemic 0 min, cold ischemic 794 min for liver and 1143 min for kidney). Bleeding during operation (about 7000 mL) mainly came from the right kidney. The haemodynamic was stable.
Immunosuppression regimen was initially based on cyclosporine. Oral cyclosporine 500 mg was given the night before operation and 1000 mg methylprednisolone was given intravenously before revascularization. However, due to the delayed recovery of function of the implanted kidney, postoperative immunosuppression protocol shifted to methylprednisolone + mycophenolate mofetil (MMF, Roche Corp.). The dosage of methylprednisolone during the 8-d post transplantation were 200, 160, 260, 220, 160, 100, 40, 20 mg separately and, maintained at a dosage of prednisolone 20 mg/d. MMF 2 g/d was given orally until the 16th d, when bone marrow suppression was manifested. Sandostatin (somatostatin, Sandoz Corp.) and Losec (Astra) were given daily after operation. Beta-lactam antibiotics, ganciclovir (Cymevene, Roche Corp.) and injection fluconazole (Diflucan, Pfizer Corp.) were used for prophylaxis of bacterial, cytomegalovirus (CMV) and fungus infections separately.
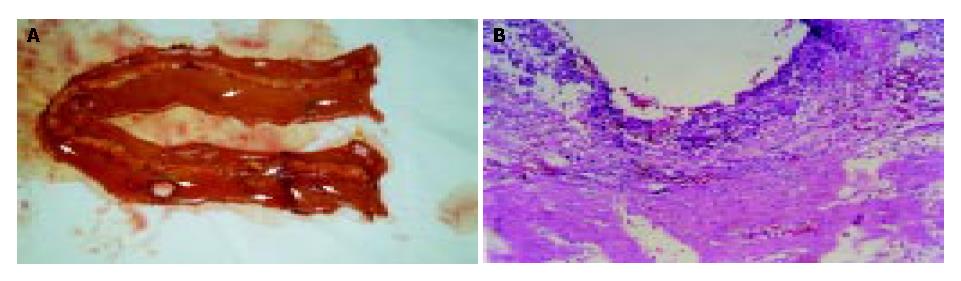
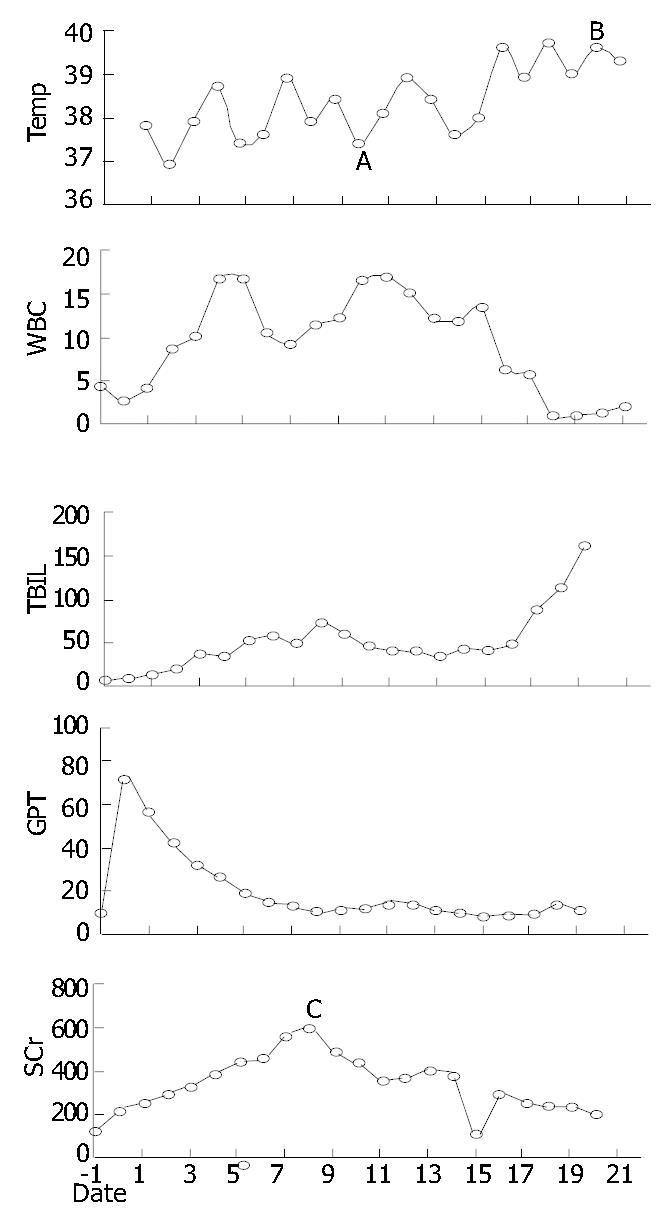
The patient woke up 2 h after returning to ICU. Due to transfusion overload (6000 mL positive balance in the first 24 h) and retention of sodium and water, acute left heart failure, pulmonary edema and ARDS presented in turn. All were reversed 6 d later. The liver function recovered well. Drainage from left side subdiaphragmatic location increased continuously. Its creatinine concentration was 2-fold higher than that of blood (1114 μmol/L vs 586 μmol/L), which indicated urine leakage from the right kidney. Since d 5 postoperatively (POD), daily urine decreased from 900 mL to 40 mL, and serum creatinine peaked to 626 mmol/L (glomerular rate 22.6 mL/min). Haemodialysis was performed daily since d 8 postoperatively. Passage of flatus and green stools began from d 6 postoperatively, and changed to water like, with frequent discharge and urgency to defecate. Temperature mounted since d 7 postoperatively. Obvious abdominal pain and distention with lower abdominal tenderness presented on d 10 postoperatively. White blood cell count rose to 17.35 × 109/L. No vascular problem of implanted liver and kidney was found in real-time ultrasound examination. In emergent exploration on d 10, two sites of bowel perforation at a diameter of 1.2 cm and 0.6 cm were noted 40-50 cm proximal to ileocecal area; and multiple ulcerative lesions with purulence were scattered on the serosa of terminal ileum. Postoperative pathology found no sign of rejection in the implanted liver and kidney except that, acute tubular necrosis in the graft kidney; acute purulence with multiple acute perforations on the ileum wall; and especially, the inflammation on the serosa side was more severe than that of the mucosa side (Figure 1). Ileum of 60 cm (30 cm proximal to the ileocecal) long was resected and an end-to-end anastomosis was done. Distention and abdominal pain were not relieved after laparotomy. And multiple ulcers appeared on the buccal mucosa, with skin rash on the forehead and chest. The temperature rose to 39.5 °C on d 15 postoperatively, accompanied with decreased counting of WBC and PLT (1.3 × 109/L, 1.8 × 109/L separately). A bone marrow aspiration showed severe marrow suppression that was not ultimately reversed by granulocyte macrophage colony stimulating factors. Culture findings included: Pseudomonas cepacia (blood, urine, secretion from trachea, and abdominal drainage), Staphylococcus haemolyticus (abdominal drainage and wound secretion), Klebsiella pneumonia, Acinetobacter junii (secretion from trachea), Candida parapsilosis, Candida sake (urine). Serology supervision of CMV, herpes simplex virus and EB virus were always negative. No reperforation was found except multiple superficial ulcers scattered on the whole bowel wall and mesentery in reexploration on d 20 postoperatively. Biopsy of graft liver/kidney and chest rash indicated no evidence of rejection and GVHD. The case progressed to death on d 21 for multiple organ failure (Data shown in Figure 2).
For liver transplantation, postoperative IP mostly happens among pediatric recipients (6.4%-20%)[1,3], especially in those who have had previous abdominal surgery but rarely seen in adults. The median delay between transplantation and perforation was 13 d, according to Soubrane O.[3]. The sites tended to locate in small intestine[4,5] and, according to Marujo, the typical intraoperative findings in whom developed the syndrome of multiple bowel perforations were pinpoint perforations in areas of normal bowel[6]. The cause remains obscure and is probably of multifactorial origin. Risk factors have been implicated as following: a previous history of abdominal operation (especially those who received Kasai operation for congenital biliary atresia), steroid therapy, CMV infection, long time duration of operation, relaparotomy for postoperative bleeding, early portal vein embolism and veno-venous bypass[3,6,7]. Incidence of reperforation was as high as 31%-53%[7,8]. Even though there was report of 100% success rescue[6] and 70% 3-year survival[3], we noticed Shaked’s report of 50% (12/24) mortality in primary perforation and 78% (7/9) in reperforation group[4].
The incidence of IP in renal transplant recipients ranged from 0.62% to 3.4%[9,10] or, in reports of larger sample, differed from 1.1% to 2.1%[11,12]. Most perforations occurred within a few weeks or months after engraftment, the period of most intense immunosuppression[9,12]. The pathogenesis was related to a high incidence of diverticular disease in patients with polycystic kidneys and/or chronic renal failure[11]. Other risk factors included overimmunosuppression[9,12], CMV infection[13], and the transplant procedure[11]. The average mortality rate was 56.5%[14]. This high mortality appeared to be related to the effects of immunosuppression and associated response to sepsis[9]. Clinical findings in these patients, such as fever or leucocytosis, might be masked by the immunosuppressive agents[12]. And pneumoperitoneum on abdominal roentgenograms was not necessarily positive[1,4]. Therefore, prompt diagnosis, aggressive surgical care consisting of resectional therapy, broad-spectrum antibiotics, and a reduced immunosuppressive protocol are crucial to outcome[2].
In our case, without a previous history of abdominal surgery and consequent adhesion, resections of the original liver and left kidney as well as implantation of graft liver and kidney were smooth, although the whole duration of operation lasted 18 h due to the limitation of personnel in the renal transplantation group. Diverticulosis and CMV infection were also cautiously excluded by inspection during operation and postoperative supervision separately. Furthermore, no pathologic evidence of GVHD was found in the examinations of either the perforated ileum or biopsy skin rash in this case. In our case, it is hard to blame just the absence of venovenous bypass during the anhepatic phase as the only risk factor in inducing gastrointestinal congestion thus responsible for the perforation[6]. But the postoperative sodium and water retention aggravated the bowel edema and stasis authentically. In addition, an obvious risk factor herein, is the relatively high dose of steroid that has not tapered down routinely because of the withdrawal of cyclosporine. Overimmunosuppression may increase risk of IP through inducing damage to the barrier of intestinal mucosa[9,12]. Thus we propose that, the long duration of operation without venovenous bypass, overdose of steroid together with postoperative volume excess may all contribute to the risk of idiopathic multiple IPs in the present case.
Also, we suggest that intraperitoneal infection in this case might originate first from the infected cyst of the fenestrated kidney and might be prior to the occurrence of IP, based on the following evidences: (1) Neutrophils counting was above normal before operation. (2) G- bacillus was found in the fluid from left cysts during operation. (3) Evidence of urine leakage from the right kidney was present. (4) The inflammation on the serosa side was more severe than that of the mucosa side under pathologic inspection. This supports Jeyarajah’s observation in 6 cases of CPCD who received liver and kidney transplantation and that, possibly due to overimmunosuppression or the presence of infectious foci in residual cysts, these patients seemed to be very susceptible to infectious complications after transplantation[15]. Unfortunately, the intraperitoneal infection of this case was masked and deteriorated by the post-transplant leukopenia. The cause of marrow suppression in this case might be multifactorial but most likely to be drug-induced. Leukopenia and/or thrombocytopenia, the frequent side effects of MMF, ganciclovir and beta-lactam antibiotics that had been applied in this case, might be accentuated by her renal dysfunction.
In summary, the lesson we learn from this particular case is, for the CPCD, any sign of suspect renal infection found before or during LKTx is indication for the excision of original kidney and, the intensity of immunosuppression therapy should be controlled with caution.
Edited by Chen WW Proofread by Zhu LH and Xu FM
| 1. | Beierle EA, Nicolette LA, Billmire DF, Vinocur CD, Weintraub WH, Dunn SP. Gastrointestinal perforation after pediatric orthotopic liver transplantation. J Pediatr Surg. 1998;33:240-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Pirenne J, Lledo-Garcia E, Benedetti E, West M, Hakim NS, Sutherland DE, Gruessner RW, Najarian JS, Matas AJ. Colon perforation after renal transplantation: a single-institution review. Clin Transplant. 1997;11:88-93. [PubMed] |
| 3. | Soubrane O, el Meteini M, Devictor D, Bernard O, Houssin D. Risk and prognostic factors of gut perforation after orthotopic liver transplantation for biliary atresia. Liver Transpl Surg. 1995;1:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Shaked A, Vargas J, Csete ME, Kiai K, Jurim O, Colquhoun S, McDiarmid SV, Ament ME, Busuttil RW. Diagnosis and treatment of bowel perforation following pediatric orthotopic liver transplantation. Arch Surg. 1993;128:994-998; discussion 994-998;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bilik R, Yellen M, Superina RA. Surgical complications in children after liver transplantation. J Pediatr Surg. 1992;27:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Marujo WC, Stratta RJ, Langnas AN, Wood RP, Markin RS, Shaw BW. Syndrome of multiple bowel perforations in liver transplant recipients. Am J Surg. 1991;162:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Vilca Melendez H, Vougas V, Muiesan P, Andreani P, Mieli-Vergani G, Rela M, Heaton ND. Bowel perforation after paediatric orthotopic liver transplantation. Transpl Int. 1998;11:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Yamanaka J, Lynch SV, Ong TH, Balderson GA, Strong RW. Posttransplant gastrointestinal perforation in pediatric liver transplantation. J Pediatr Surg. 1994;29:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Bardaxoglou E, Maddern G, Ruso L, Siriser F, Campion JP, Le Pogamp P, Catheline JM, Launois B. Gastrointestinal surgical emergencies following kidney transplantation. Transpl Int. 1993;6:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Morcillo Rodenas MA, García Espinosa R, Moliner Quiles C, Pallardo Mateu L, Planells Roig M, Rodero Rodero D. [Colonic perforation in patients with kidney transplant]. Rev Esp Enferm Dig. 1990;77:49-51. [PubMed] |
| 11. | Church JM, Fazio VW, Braun WE, Novick AC, Steinmuller DR. Perforation of the colon in renal homograft recipients. A report of 11 cases and a review of the literature. Ann Surg. 1986;203:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Stelzner M, Vlahakos DV, Milford EL, Tilney NL. Colonic perforations after renal transplantation. J Am Coll Surg. 1997;184:63-69. [PubMed] |
| 13. | Toogood GJ, Gillespie PH, Gujral S, Warren BF, Roake JA, Gray DW, Morris PJ. Cytomegalovirus infection and colonic perforation in renal transplant patients. Transpl Int. 1996;9:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Lin HS, Yang CR, Chang CH, Chang CL, Wu HC, Ho HC. Bowel perforation--a fatal complication following renal transplantation: a report of two cases. Zhonghua Yixue Zazhi (Taipei). 1994;54:442-446. [PubMed] |
| 15. | Jeyarajah DR, Gonwa TA, Testa G, Abbasoglu O, Goldstein R, Husberg BS, Levy MF, Klintmalm GB. Liver and kidney transplantation for polycystic disease. Transplantation. 1998;66:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |