Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2747
Revised: January 5, 2004
Accepted: January 12, 2004
Published online: September 15, 2004
AIM: To establish an ELISA kit using monoclonal antibodies against Clostridium difficile (C. difficile) toxin A.
METHODS: An indirect sandwich ELISA was described using the purified rabbit monospecific antiserum as capturing antibody. After the polystyrene microtitre plates with 96 flat-bottomed wells were coated with rabbit antiserum, the wells were blocked with 100 g/L BSA in PBS-T. C. difficile toxin A or culture filtrates were added to each well and then monoclonal antibodies IgG-horseradish peroxidase conjugate was added as detecting antibody, tetramethylbenzidine was used as substrate and A450 of the stopped reacting product was recorded in an automated plate reader.
RESULTS: The tested specimens included culture filtrates of 2 strains of toxigenic C. difficile, 2 strains of non-toxigenic C. difficile, 26 strains of E. coli, 2 strains of S. dysenteriae, 1 strain of Bif. infantis, 5 strains of V. cholera, 2 strains of S. typhi, 7 strains of C. botulinum, 1 strain of toxigenic C. sordllii, and 1 strain of C. butyricum. A total of 47 strains of culture filtrates were all negative except for 2 strains of toxigenic C. difficile. The detective limitation of toxin A was 0.1 ng/mL.
CONCLUSION: An ELISA kit with high specificity and excellent sensitivity for the rapid detection of C. difficile toxin A was established. It will be a useful tool for diagnostic test of C. difficile toxin A.
-
Citation: Fu SW, Zhang YL, Zhou DY. Development of an ELISA kit using monoclonal antibody to
Clostridium difficile toxin A. World J Gastroenterol 2004; 10(18): 2747-2749 - URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2747
Clostridium difficile (C. difficile), which has been reported as the major cause of antibiotic-associated colitis (AAC) and pseudomembranous colitis (PMC) in humans and experimental animals, produces at least two toxins, named toxin A and B. Toxin A, a tissue-damaging enterotoxin, can lead to hemorrhagic fluid accumulation in rabbit ileal loops and is cytotoxic for cultured fibroblasts. Toxin B is an extremely potent cytotoxin for many cultured cells[1,2].
Polyclonal antibodies are used in immunoassays and detection for C. difficile toxin in many clinical researches. They are against toxin A and toxin B, and may be against other antigens at the same time, causing false positive results[3-5]. The problem may be solved when monoclonal antibodies were used. They can not only improve the specificity of the method, but also decrease the false positive results as well. The present report describes an enzyme-linked immunosorbent assay (ELISA) which used monospecific antibody as a capture antibody, and monoclonal antibody as a detection antibody, for detection of C. difficile toxin A in clinical specimens.
The 49 strains of bacteria included 2 strains of toxigenic C. difficile, 2 strains of non-toxigenic C. difficile; 26 strains of E. coli, 2 strains of S. dysenteriae; 1 strain of Bif. infantis; 5 strains of V. cholera; 2 strains of S. typhi; 7 strains of C. botulinum; 1 strain of C. sordellii; and 1 strain of C. butyricum. All strains were cultured with proper medium separately and the culture filtrates were used for the assay.
Toxigenic C. difficile VPI 10463 was grown anaerobically at 37 °C for 72 h in brain heart infusion (Difco) by the dialysis bag methods. Toxin A was purified by precipitation with 500 g/L (NH4)2SO4 and acid precipitation at pH5.5, then by ion-exchange chromatography on DEAE-Toyopearl 650 mol/L.
Purified toxin A was inactivated with 4 g/L formaldehyde at 37 °C for 72 h, mixed with SEPPIC adjuvant(5.41:4.6 v/v, Montanide ISA206, France), and then injected in to a New Zealand white male rabbit of 2 kg weight every 2 wk over a period of 4 wk. Booster injections of 0.2 mg of toxoid A were prepared with adjuvant and injected subcutaneously at 4 wk intervals over a period of 32 wk, and antiserum was collected 1 wk after the final booster dose.
Purified toxin A of C. difficile (100 μg) was inactivated with 4 g/L formaldehyde at 37 °C for 72 h, and mixed with SEPPIC adjuvant, BALB/c mice were injected intraperitoneally with 0.5 mL of toxoid A at 2 wk intervals over a period of 8 wk. Three days before fusion, one mouse was boosted with the same quantity of toxoid A without the adjuvant. The splenocytes from immunized mice were fused with myeloma cells Sp2/0. The hybridoma cells were screened by indirect ELISA and cloned by limiting dilution method.
Antibodies were purified by precipitation with 400 g/L (NH4)2SO4 followed by precipitation with 330 mL/L (NH4)2SO4 for 3 times, centrifuged at 6000 r/min for 30 min, and then loaded on Sephacryl-300 chromatography column.
Sodium periodate solution (0.3 mL, 0.1 mol/L) and 5 mg horseradish peroxidase dissolved in 1 mL of water, the mixture was stirred for 30 min at 4 °C and dialyzed at 4 °C against 0.01 mol/L sodium acetate buffer (pH4.4) overnight, followed by addition of 0.5 mL of 0.16 mol/L ethylene glycol, stirred for 1 h at 4 °C. Monoclonal IgG of 5 mg in 1 mL of 0.05 mol/L sodium carbonate buffer (pH9.6) was added immediately, stirred and dialyzed at 4 °C with same buffer overnight before any unconjugated enzyme was removed by addition of 0.2 mL sodium borohydride solution (5 mg/mL, 4 °C, 3 h), then precipitated by addition of equal volumes of 100% ammonium sulfate (4 °C, 2.5 h), centrifuged (6000 r/min, 15 min), dialyzed against 0.01 mol/L PBS (pH7.4) at 4 °C overnight, and stored at 4 °C after diluted with 500 mL/L glycerol (1:1).
Ninety-six-well polystyrene flat-bottomed microtitre plates were coated with 100 μL of purified rabbit monospecific antitoxin (8 μg/mL, capture antibody) in 0.05 mol/L carbonate buffer (pH9.6) and incubated overnight at 4 °C, the plates were washed once in PBS-T (0.01 mol/L PBS containing 0.5 g/L Tween-20, pH7.4). After 200 µL of 100 g/L BSA in PBS-T was added to the wells and incubated at 37 °C for 2 h, washed 5 times in PBS-T with 3 min incubation at room temperature between each wash, 100 µL of C. difficile toxin A or test samples in PBS-T were added to each well and incubated for 1 h at 37 °C, washed for 5 times. Then 100 µL of 1 :1000 diluted monoclonal antibodies IgG-horseradish peroxidase conjugate (detecting antibody) was added for 1 h at 37 °C, wells were washed 5 times with PBS-T, and 0.1 mL of TMB(3,3’,5,5’-tetramethylbenzidine) substrate was added to each well. After 15 min at 37 °C in the dark, the reaction was stopped by the addition of 1 drop of 2 mol/L sulfuric acid and A450 was measured. ELISA titers of positivity were expressed as A450 >/= 0.10. Non. toxigenic C. difficile culture filtrate was used as negative control.
Protein concentration was determined by the method of Coomassie brilliant blue G-250, and bovine serum albumin was purchased from Sigma Company of USA.
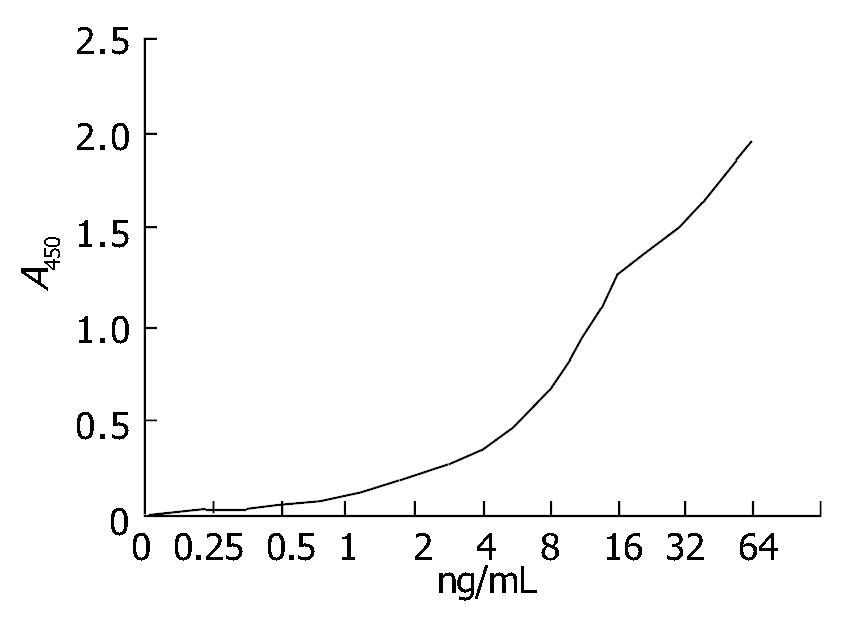
Six hybridoma cell lines (2H7, 3E9, 4B5, 5C10, 6G8 and 8A1) secreting mAbs against C. difficile toxin A were produced. The Ig subclasses of mAbs 2H7, 3E9 and 6G8 were IgM, mAbs 4B5 and 8A1 were IgG1, mAb 5C10 was IgG2a. All 6 mAbs had no neutralization activity. Epitope recognized by 5 mAbs (2H7, 4B5, 5C10, 6G8 and 8A1) differed from mAb 3E9. Relative affinities of mAbs 8A1 and 4B5 were all above 105, and those of the other 4 mAbs were 104. mAbs 8A1 were conjugated to horseradish peroxidase for ELISA, A403/A280 was 0.40 and the optimal dilution was 1:1000. The monospecific antiserum could neutralize all activities of C. difficile toxin A. The ELISA titer was 106/mL. (Figure 1)
Purified C. difficile toxin A was detected with ELISA and a high sensitivity of 0.1 ng/mL was found.
The tested specimens were from culture filtrates of 49 strains. Forty-seven strains were all negative except that 2 strains were positive. Table 1 illustrates the ELISA results with a high specificity.
| Strain | Number | Culture supernatant | |
| Positive (n) | Negative (n) | ||
| C. difficile toxigenic | 2 | 2 | 0 |
| C. difficile non-toxigenic | 2 | 0 | 2 |
| E. coli | 26 | 0 | 26 |
| S. dysenteriae | 2 | 0 | 2 |
| Bif. infantis | 1 | 0 | 1 |
| V. cholera | 5 | 0 | 5 |
| S. typhi | 2 | 0 | 2 |
| C. botulinum | 7 | 0 | 7 |
| C. sordellii | 1 | 0 | 1 |
| C. butyricum | 1 | 0 | 1 |
Ninety-six-well polystyrene flat-bottomed microtitre plates were coated with 100 μL of purified rabbit monospecific antitoxin (8 μg/mL) in carbonate buffer (pH9.6) and incubated overnight at 4 °C, the plates were washed once in PBS containing 0.5 g/L Tween-20, pH7.4 (PBS-T). After 200 μL of 100 g/L BSA in PBS-T was added to the wells and incubated at 37 °C for 3 h, and then vacuumed and kept in plastic bag at 37 °C for 6 d, the titer was detected, indicating that the kit can be kept at 4 °C for at least 1 year.
A number of rapid (enzyme immunoassay and latex agglutination) and conventional (direct plating and tissue culture) tests have been developed as aids in the diagnosis of C. difficile infection: (1) Tissue culture cytotoxicity assay is the best available laboratory test for determination of the role of C. difficile in the pathology of a given patient diarrhea, it has an excellent sensitivity and specificity, but its utility is limited because of its inherent technical complexity, time requirement, specimen-handling requirements, and high costs; (2) Latex agglutination test was found to be nearly as sensitive as the cytotoxicity assay and it did not detect toxin A but a C.difficile cell-associated protein; (3) Organism culture: first, the asymptomatic carriage rate of this organism may be as high as 20% in patients receiving antibiotics making interpretation of positive culture data difficult. Second, organisms that do not produce toxin are thought to be avirulent. Isolates must be proved to produce toxin to be considered pathogenic; (4) ELISA. ELISA has the potential of greatly simplifying and improving the efficiency of the laboratory diagnosis of C. difficile associated diseases. It has the high sensitivity and excellent specificity comparable with tissue culture cytotoxicity assay. Using the commercial enzyme immunoassays for the detection of toxin A or toxin B, the results could be available in 2-4 h. ELISA was a perfect method to detect toxin A in feces[7,8].
The advantage of ELISA for detection is that: first, as a clinical expression of C. difficile infection, toxin A was more stable than toxin B. Second, toxin A was easy to be purified and its antibodies were easy to produce. Third, the monoclonal antibodies can be prepared in unlimited quantities and are more reproducible reagents than other antibodies. After comparing the antibody preparations by indirect ELISA, Lyerly et al[8] reported that ELISA with monoclonal antibodies could detect 4 ng of toxin A, but the polyclonal antibodies purified by affinity chromatography and monospecific antiserum could detect 1 ng of toxin A. Laughon et al[9] reported that they could detect 0.1 ng (1.0 ng/mL) of toxin A with a monospecific antiserum. In this study we present ELISA kits which used the purified rabbit monospecific antitoxin as capturing antibody and monoclonal antibodies IgG-horseradish peroxidase conjugate as detecting antibody, the detective limitation of toxin A was 0.1 ng/mL.
Antibiotic-associated diarrhea and PMC are the major problems in a variety of health care settings. Requests for the laboratory diagnosis of C. difficile-associated diseases are frequently made, but there have been no diagnostic reagents in China until now. Thus, rapid diagnosis of C. difficile in patients with PMC and antibiotic-associated diarrhea is very important and guides both the treatment and control of nosocomial spread of infection. The ELISA kits with a high specificity, sensitivity and stability for the rapid detection of C. difficile toxin A are presented here. The usefulness of the ELISA kits awaits further studies in clinic.
Edited by Chen WW and Zhu LH Proofread by Xu FM
| 1. | Bartlett JG. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 256] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18 Suppl 4:S265-S272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Schué V, Green GA, Monteil H. Comparison of the ToxA test with cytotoxicity assay and culture for the detection of Clostridium difficile-associated diarrhoea disease. J Med Microbiol. 1994;41:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Krishnan C. Detection of Clostridium difficile toxins by enzyme immunoassay. J Hyg (Lond). 1986;96:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Merz CS, Kramer C, Forman M, Gluck L, Mills K, Senft K, Steiman I, Wallace N, Charache P. Comparison of four commercially available rapid enzyme immunoassays with cytotoxin assay for detection of Clostridium difficile toxin(s) from stool specimens. J Clin Microbiol. 1994;32:1142-1147. [PubMed] |
| 6. | Jie Y, Luo HB, Lu DY. Modern microbiological techology and its application 1st edition, people health press.. Beijing. 1997;173-183. |
| 7. | Lyerly DM, Phelps CJ, Wilkins TD. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985;21:12-14. [PubMed] |
| 8. | Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1-18. [PubMed] |
| 9. | Laughon BE, Viscidi RP, Gdovin SL, Yolken RH, Bartlett JG. Enzyme immunoassays for detection of Clostridium difficile toxins A and B in fecal specimens. J Infect Dis. 1984;149:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.0] [Reference Citation Analysis (0)] |