Published online Sep 15, 2004. doi: 10.3748/wjg.v10.i18.2727
Revised: January 6, 2004
Accepted: January 12, 2004
Published online: September 15, 2004
AIM: To assess the efficacy and safety of intraperitoneal chemotherapy in patients undergoing curative resection for gastric cancer through literature review.
METHODS: Medline (PubMed) (1980-2003/1), Embase (1980-2003/1), Cancerlit Database (1983-2003/1) and Chinese Biomedicine Database (1990-2003/1) were searched. Language was restricted to Chinese and English. The statistical analysis was performed by RevMan4.2 software provided by the Cochrane Collaboration. The results were expressed with odds ratio for the categorical variables.
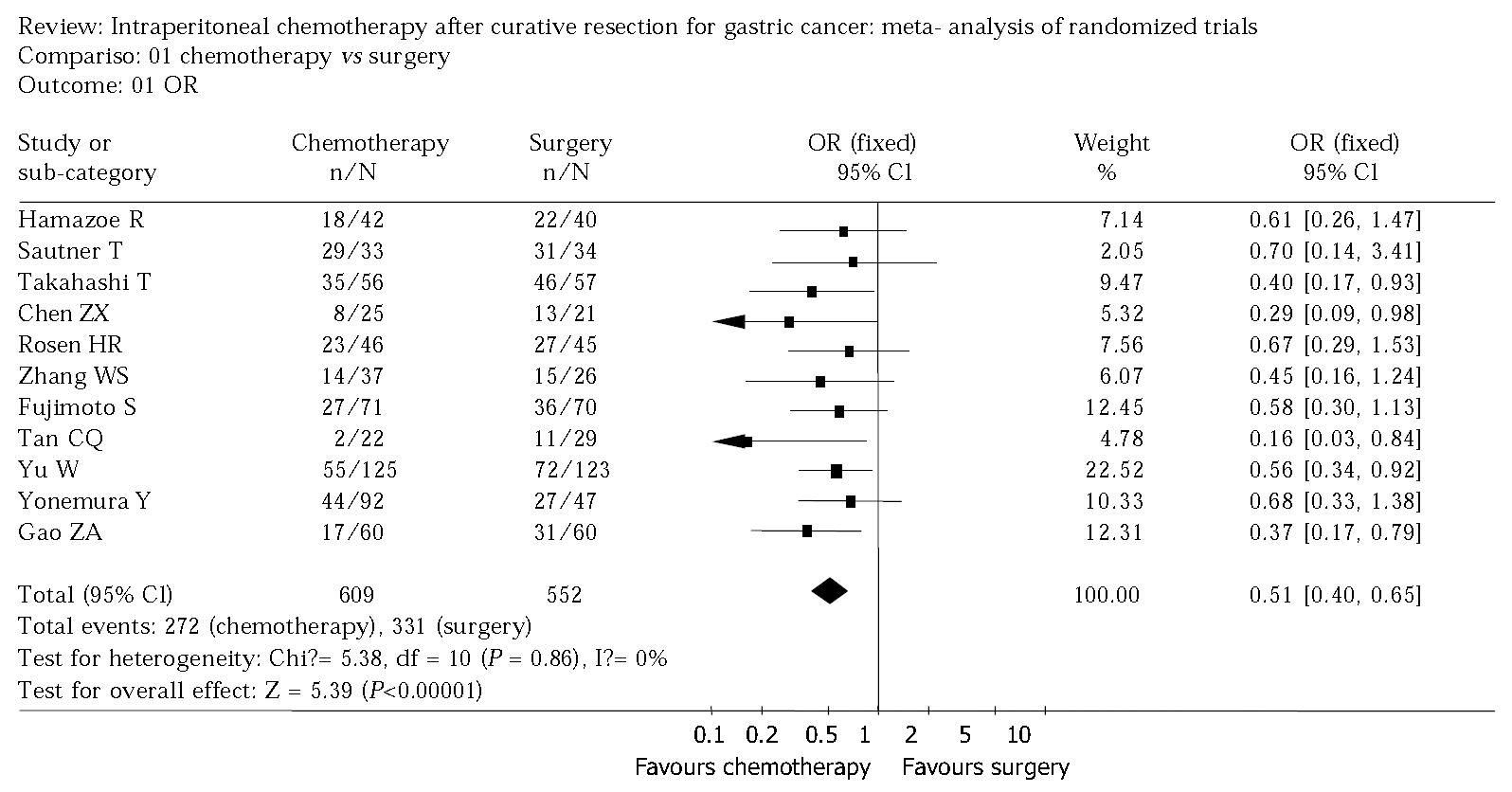
RESULTS: Eleven trials involving 1161 cases were included. The pooled odds ratio was 0.51, with a 95% confidence interval (0.40-0.65). Intraperitoneal chemotherapy may benefit the patients after curative resection for locally advanced gastric cancer, and the combination of intraperitoneal chemotherapy with hyperthermia or activated carbon particles may provide more benefits to patients due to the enhanced antitumor activity of drugs. Sensitivity analysis and fail-safe number suggested that the result was comparatively reliable. However, of 11 trials, only 3 studies were of high quality.
CONCLUSION: Intraperitoneal chemotherapy after curative resection for locally advanced gastric cancer may be beneficial to patients. Continuous multicenter, randomized, double blind, rigorously designed trials should be conducted to draw definitive conclusions.
- Citation: Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004; 10(18): 2727-2730
- URL: https://www.wjgnet.com/1007-9327/full/v10/i18/2727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i18.2727
Gastric cancer is the second most common cause of cancer death worldwide. Surgery is the main choice of radical treatment, however, even for patients with apparently completely resection, the five-year survival rate is about 30%-60%, which has been disappointing. Peritoneal metastasis is the most common type of recurrence after surgery and has the worst prognosis in patients with advanced stomach cancer. To improve the survival of the patients with gastric cancer, intraperitoneal chemotherapy (IPT) as a possible effective treatment for peritoneal dissemina-tion in locally advanced gastric cancer has been investigated clinically, but the results among these studies are still different and disputed. To evaluate whether IPT benefited the patients undergoing radical resection for locally advanced gastric cancer, we summarized all the available randomized trials and combined the results for meta-analysis.
Medline (PubMed) (1980-2003/1), Embase (1980-2003/1), Cancerlit Database (1983-2003/1) and Chinese Biomedicine Database (1990-2003/1) were searched. Languages were restricted to Chinese and English.
To be included, trials had to be randomised and controlled. Trials could be single-blind, double-blind or not blind. Chemotherapy groups were treated intraperitoneally, including intraperitoneal chemotherapy with or without activated carbon particles (CH), intraperitoneal hyperthermic perfusion. No oral or intravenous chemotherapy, chemoimmunotherapy, or radiotherapy were used. Those receiving gastrectomy alone were included in control group. All patients must have had a potential curative surgery for locally advanced gastric cancer.
Data extracted included the baseline data of intraperitoneal chemotherapy group and control group, assessments of eligibility and trial quality, number of survival and death and the statistical consideration.
Data collection The content terms of stomach neoplasms, intraperitoneal chemotherapy and surgery, and the methodological terms of clinical trial, phase III, randomized trial, double blind method were used. These searches were supplemented by hand searching of the reference lists of identified trials and review articles. Two reviewers assessed independently the outcome data using a predesigned strategy. They also evaluated study quality using Jadadscale[1] plus allocation concealment. Intention-to-treat analyses were also performed. Agreement was achieved through discussion when they have different evaluations.
The results of eligible trials were pooled using RevMan4.2 software provided by the Cochrane Collaboration. The result was expressed with odds ratio (OR) for the categorical variables. Q statistic test was used for gross statistical heterogeneity. Sensitivity analysis was performed by excluding the trials in which Jadad-scale was low. Publication bias was assessed by calculating the Rosenthal s fail-safe number. Subgroup analysis was performed by collecting summaries for subsets of studies with different characteristics.
There was good agreement between the two reviewers on the eligibility, quality scores and data extraction of analysis. Eighteen random trials including surgery plus intraperitoneal chemotherapy, preliminary surgery alone were analysed, from which 7 reports were excluded for repetitive studies. Table 1 shows the details of the 11 trials[2-12] included in the analysis with a total enrollment of 1161 patients, in which 609 patients were assigned to the treatment group and 552 to the control group. The average sample size was 106 patients. Of 11 trials, three studies were of high quality according to the Jadad-scale (with three scores), one trial mentioned double-blind design and sample-size calculation, and two studies described intention-to-treat analysis. The fail-safe number of 104 suggested that no important publication bias existed in this meta-analysis.
| Author | Country | Publication time | Chemotherapy regimens | IPT | Surgery | Follow-up (mo) | Quality score |
| (number of death/total) | |||||||
| Rosen HR | Austria | 1998 | MMC+Carbon | 23/46 | 27/45 | 36 | 4 |
| Sautner T | Austria | 1994 | Cisplatin | 29/33 | 31/34 | 60 | 3 |
| Takahashi T | Japan | 1995 | MMC+Carbon | 35/56 | 46/57 | 36 | 3 |
| Yu W | Korea | 2001 | MMC+5-Fu | 55/125 | 72/123 | 60 | 2 |
| Yonemura Y | Japan | 2001 | MMC+CDDP | 44/92 | 27/47 | 60 | 1 |
| Hamazoe R | Japan | 1993 | MMC | 18/42 | 22/40 | 60 | 1 |
| Chen ZX | China | 1996 | DDP | 8/25 | 13/21 | 18 | 1 |
| Zhang WS | China | 1998 | 5-Fu | 14/37 | 15/26 | 36 | 1 |
| Fujimoto S | Japan | 1998 | MMC | 27/71 | 36/70 | 96 | 1 |
| Tan CQ | China | 2000 | MMC | 2/22 | 11/29 | 36 | 1 |
| Gao ZA | China | 2002 | MMC+DDP+HCPT | 17/60 | 31/60 | 36 | 2 |
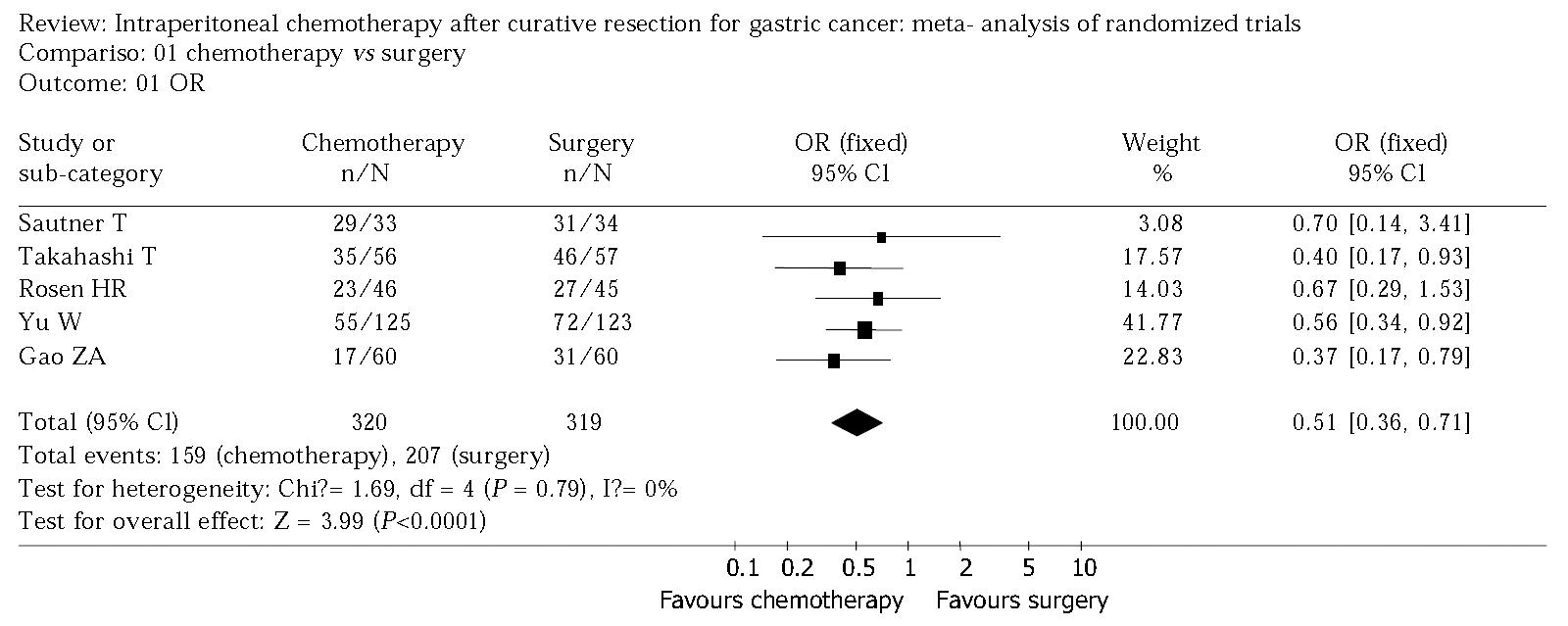
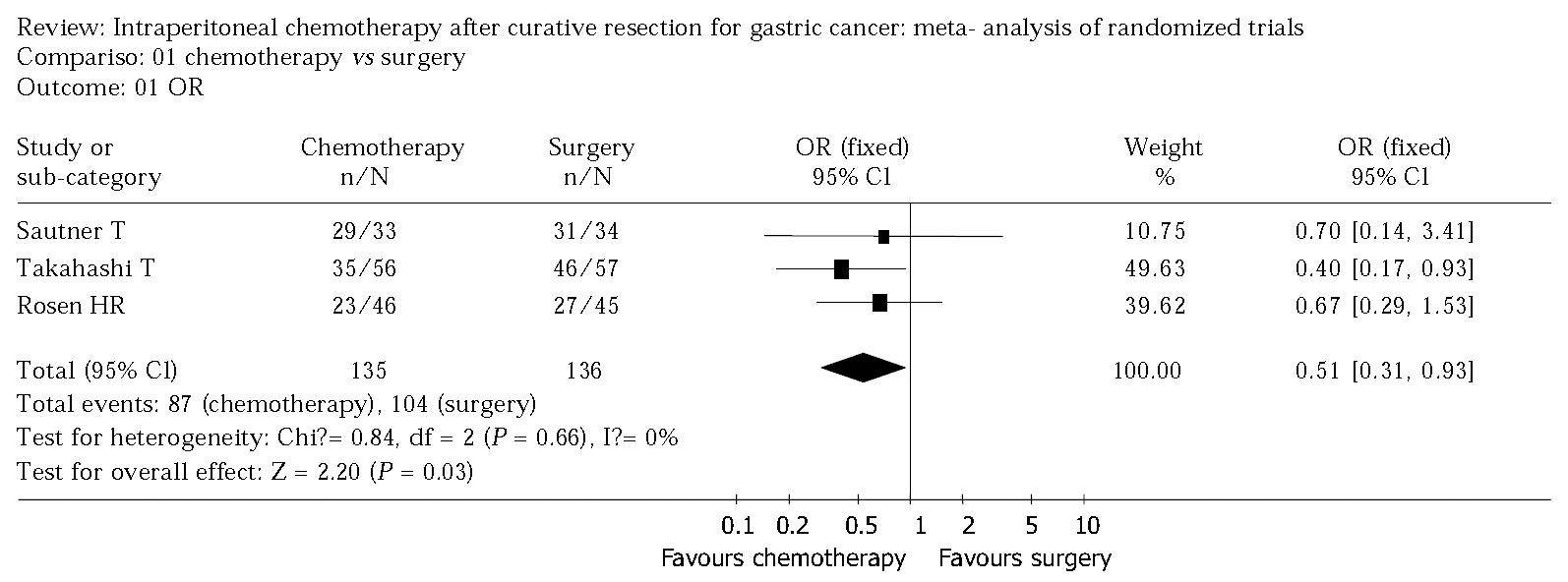
Figure 1 shows the result of the meta-analysis. There was no statistically significant heterogeneity in our analysis, so a fixed effect model was used and the odds radio was 0.51 (95%CI 0.40 to 0.65). The sensitivity analysis was performed by excluding trials with Jadad-scale scores between 1 and 3 and revealed the same difference between intraperitoneal chemotherapy and surgery alone (odds ratio 0.51, 95%CI 0.36 to 0.71: odds ratio 0.54, 95%CI 0.31 to 0.93) (Figure 2, Figure 3).
As shown in Table 2, intraperitoneal hyperthermic chemoperfusion (IHCP) or chemotherapy with activated carbon particles (CH) produced more benefits to patients than those without hyperthermia or CH; the group of trials from Asian countries exhibited a trend towards a more significant effect than those from non-Asian countries, which showed no effect with IPT; for trials with more than 5 years of follow-up, the effects were less obvious than those of shorter follow-up, if indicated that IPT might afford long-term survival by delaying relapse and recurrence.
| Characteristic | No. of trials | OR | 95%CI |
| IPT (without CH and IHCP) | 2 | 0.57 | (0.35-0.92) |
| IPT (only with CH) | 2 | 0.52 | (0.29-0.94) |
| IHCP | 7 | 0.48 | (0.35-0.67) |
| Follow-up time (mo) < 60 | 6 | 0.40 | (0.27-0.59) |
| Follow-up time (mo) ≥ 60 | 5 | 0.60 | (0.44-0.82) |
| Asia | 9 | 0.49 | (0.38-0.64) |
| Non-Asia (Austria) | 2 | 0.67 | (0.32-1.41) |
All studies described the side effects of medicine, including anastomotic leakage, leukocytopenia, fever, intestinal obstruction, fistula, intraabdominal bleeding, and prolonged abdominal pain, etc. Of 11 trials, 5 had mild complications; 3 had no significant differences in adverse effects between the surgery group and the chemotherapy group; 2 trials produced complications in the chemotherapy group: one was bowel fistula, the other was intraabdominal abscess which turned better after effective treatment. One trial from Austria reported serious side effects by IPT, the complications of the treatment group and control group were respectively 35% and 16%, the death rates, 11% and 2%, which terminated the trial ahead of schedule.
IPT is applied to kill residual tumor cells left behind during surgery, which can not be achieved by the intravenous approach, especially for stage 3 and 4 gastric carcinomas. It is of much importance to extirpate the free tumor cells in the abdominal cavity and micrometastases on the peritoneal surface to attain longer survival. Animal trials and phase II clinical trials have revealed that IPT could be effective to prevent peritoneal dissemination and liver metastasis[13].
The aim of meta-analysis is to provide a comprehensive, up-to-date summary of average effect of all the relevant randomized controlled trials, to provide reliable guidance for clinical practice and future research[14]. Based on our results, IPT may benefit the patient after curative resection for locally advanced gastric cancer, and the combination of IPT with hyperthermia or activated carbon particles may provide more benefit to patients due to the enhanced antitumor activity of drugs[15]. Sensitivity analysis and fail-safe number suggested that the results were comparatively reliable.
Two trials from Austria showed that intraperitoneal chemotherapy was not beneficial to patients, one of them terminated ahead of time because of serious adverse effects. Nine Asian studies (from China, Japan, Korea) confirmed a significant survival benefit for patients with tolerable side effects of antitumor drugs. Considering the difference between western studies and Asian studies[16,17], we speculate that they may have different aetiology or biology methods[18].
The adverse effect was an important factor to influence the result of intraperitoneal chemotherapy. However, only 4 trials described the side effects of medicine on the basis of World Health Organization classification, and the different forms of illustration made it difficult to analyze the effect according to evidence based medicine. There fore, we should observe and describe the toxicity of medicine by WHO standard in future clinical research.
Of included 11 trials, only 3 trials were of high quality (Jadad-scale with 3 scores) and the other 8 trials were of low quality, which weakened our evidence. On the other hand, reliance on published trials alone might distort the outcome of meta-analysis, because positive studies were more likely to be published than negative ones.
At present, the treatment effect of gastric cancer is still disappointing. Many surgeons hold that the stomach cancer patients cannot benefit from IPT, which is different from our meta-analysis results. To draw definitive conclusions, move effective multicenter, randomized, double blind, rigorously designed trials are needed.
Edited by Chen WW and Zhu LH Proofread by Xu FM
| 1. | Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 752] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy of advanced gastric cancer: results of a prospective randomized trial. World J Surg. 2001;25:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Rosen HR, Jatzko G, Repse S, Potrc S, Neudorfer H, Sandbichler P, Zacherl J, Rabl H, Holzberger P, Lisborg P. Adjuvant intraperitoneal chemotherapy with carbon-adsorbed mitomycin in patients with gastric cancer: results of a randomized multicenter trial of the Austrian Working Group for Surgical Oncology. J Clin Oncol. 1998;16:2733-2738. [PubMed] |
| 4. | Takahashi T, Hagiwara A, Shimotsuma M, Sawai K, Yamaguchi T. Prophylaxis and treatment of peritoneal carcinomatosis: intraperitoneal chemotherapy with mitomycin C bound to activated carbon particles. World J Surg. 1995;19:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R. Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol. 1994;12:970-974. [PubMed] |
| 6. | Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer. 1994;73:2048-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776-1782. [PubMed] |
| 9. | Chen ZX, Hu JK, Chen Z, Cheng JP, Peng DX, Tao QP. Positive results of therapy of gastric cancer patients after operation by intraperitoneal hyperthermic chemotherapy. Zhonghua Shiyong Yixue. 2001;3:39-40. |
| 10. | Gao ZA, Jiang ZX, Zhou F. Clinical survey of early intraperitoneal hyperthermic chemoperfusion for gastric cancer patients after operation. Zhongguo Zhongliu Linchuang. 2002;29:294-295. |
| 11. | Tang CQ, Wang XJ, Xu YK, Jiang BN, Zeng FH. Study for prevention of peritoneal dissemination of advanced gastric cancer by intraperitoneal thermochemotherapy. Shiyong Zhongliu Zazhi. 2000;15:165-167. |
| 12. | Zhang WS, Su DM, Wang K, Yi PT, Zhou HT, Guo JJ. Clinical study in prophylactic use of intraperitoneal hyperthermic chemoperfusion for the prevention of peritoneal metastasis in patients with advanced gastric carcinoma. Shanxi Yiyao Zazhi. 1998;27:67-69. |
| 13. | Devita VT, Hellman S, Rosenberg SA. Cancer principles practice of oncology (6th edition). (USA) Philadelphia:. Lippincott Williams wilkins Press. 2001;1109-1110. |
| 14. | Li Jing, Wang JL. Method and principle of systematic reviews. Zhonghua Yixue Zazhi. 2001;81:53-55. |
| 15. | Teicher BA, Kowal CD, Kennedy KA, Sartorelli AC. Enhancement by hyperthermia of the in vitro cytotoxicity of mitomycin C toward hypoxic tumor cells. Cancer Res. 1981;41:1096-1099. [PubMed] |
| 16. | Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1069] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 17. | Shimada K, Ajani JA. Adjuvant therapy for gastric carcinoma patients in the past 15 years: a review of western and oriental trials. Cancer. 1999;86:1657-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Gulmann C, Hegarty H, Grace A, Leader M, Patchett S, Kay E. Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via the retinoblastoma pathway. World J Gastroenterol. 2004;10:17-21. [PubMed] |