Published online Aug 1, 2004. doi: 10.3748/wjg.v10.i15.2205
Revised: February 23, 2004
Accepted: March 12, 2004
Published online: August 1, 2004
AIM: To investigate the signal pathway of honokiol-induced apoptosis on human colorectal carcinoma RKO cells and to evaluate whether p53 and p53-related genes were involved in honokiol-treated RKO cells.
METHODS: Cell cycle distribution and subdiploid peak were analyzed with a flow cytometer and DNA fragment with electrophoresis on agarose gels. Transcriptional level of Bax, Bcl- 2, Bid and Bcl-xl was accessed by RT-PCR. Western blotting was used to measure p53 protein expression and other factors related to apoptosis. Proliferation inhibition of two cell lines (RKO, SW480) with high expression of p53 and one cell line with p53 negative expression (LS180) was monitored by MTT assay.
RESULTS: Honokiol induced RKO cell apoptosis in a dose-dependent manner. The mRNA expression level and protein level of Bid were up -regulated while that of Bcl-xl was down-regulated, but no changes in Bax and Bcl-2 were observed. Western blotting showed p53 expression had no remarkable changes in honokiol-induced RKO cell apoptosis. LS180 cells treated with honokiol exhibited apparent growth inhibition like RKO cells and Sw480 cells.
CONCLUSION: Honokiol can induce RKO cells apoptosis through activating caspase cascade by p53-indepenent pathway.
- Citation: Wang T, Chen F, Chen Z, Wu YF, Xu XL, Zheng S, Hu X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J Gastroenterol 2004; 10(15): 2205-2208
- URL: https://www.wjgnet.com/1007-9327/full/v10/i15/2205.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i15.2205
Honokiol is a major active constituent extracted from the bark of Magnolia officianlis (Chinese name for Houpu) (Figure 1). It has a variety of pharmacological effects, such as anti-inflammatory[1], antithrombosis[2], anti-arrhythmic[3], antioxidant[4] and anxiolytic effects[5]. Recently, honokiol has been reported to exhibit a potent cytotoxic activity by inducing cell apoptosis in some cell lines[6-8] . Honokiol-triggered apoptotic process is accompanied with down- modulation of Bcl-XL, release of mitochondrial cytochrome C in CH27 cells[9].
Cells undergoing apoptosis are characterized by distinct biochemical and morphological changes. Many of the biochemical and morphological events of apoptosis are a direct result of caspase-mediated cleavage of specific substrates[10,11]. Together with caspase, Bcl-2 family is involved in inducing cell apoptosis and identified as essential components of the intracellular apoptotic signaling pathways. Cell viability versus death is determined by the relative abundance of various members of the Bcl-2 family, acting in concert with other proteins in the death pathway[12,13].
In many tumor cells, wild-type p53 is considered to participate in apoptosis in response to DNA damage[14]. P53 may transactivate apoptotic regulators, such as Bcl-2[15-17] and Bax[18,19]. Recent studies have shown that p53 plays a role in apoptosis by the mitochondrial-mediated apoptotic pathway[20]. Activation of p53 upregulates Bax[19] and increases the ratio of Bax:Bcl-2, releases cytochrome C and other polypeptides from the intermembrane space of mitochondria into cytoplasm. Cytochrome C activates caspase cascade[12,13].
It is well known that a broad range of agents can induce tumor cell apoptosis through p53-regulated manner by Bax/ mitochondria/caspase-9 pathway. But up to now, it is not clear that whether wild-type p53 takes part in honokiol-induced apoptosis. In this present study we examined whether wild-type p53 and p53-related gene were involved in honokiol-treated RKO cells.
RPMI 1640 medium was obtained from Gibco BRL. Newborn bovine serum was supplied by Sijiqing Biotechnology Co. (Hangzhou, China). Monoclonal antibodies to Bax, Bcl-xl, Bid, Bcl-2, β -actin and p53 were purchased from NeoMarkers, Fremont, CA, USA. Honokiol was got from the National Institute for Pharmaceutical and Biological Products, Beijing, China. The drug was dissolved in DMSO with the stock concentration of 10 mg/mL. It was further diluted in culture medium with the final DMSO concentration <1%. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were purchased from Sigma Chemical Corporation, USA.
Human colorectal cell lines RKO, SW480 and LS180 were provided by Cancer Institute of Zhejiang University. Three cells were maintained in RPMI 1640 medium (Gibco BRL) supplemented with 100 mL/L heat-inactivated fetal bovine serum (Si-Ji-Qing Biotechnology Co, Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a 50 mL/L CO2 atmosphere.
To detect DNA fragments, RKO cells were collected and lysed with lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 20 mmol/L EDTA, and 10 g/L NP-40. Then 10 g/L SDS and RNase (5 µg/mL) were added to the supernatants, and incubated at 56 °C for 2 h, followed by incubation with proteinase K (2.5 µg/mL) at 37 °C for 2 h. After DNA was precipitated by addition of both ammonium acetate (3.3 mol/L) and ethanol (995 mL/L) dissolved in a loading buffer, DNA fragmentation was detected by electrophoresis on 15 g/L agarose gels and visualized with ethidium bromide staining.
Honokiol-treated RKO cells (5, 10, 15 µg/mL) and vehicle were fixed with 700 mL/L alcohol for 15 min at 4 °C, then stained with 1.0 µg/mL propidium iodide (PI, Sigma, USA). The red fluorescence of DNA-bound PI in individual cells was measured at 488 nm with a FACSCalibur (Becton Dickinson, USA) and the results were analyzed using ModFit 3.0 software. Ten thousand events were analyzed for each sample.
RKO cells 1 × 105 were seeded on 24-well plate. After 24-h culture, cells were treated with 5, 10 µg/mL honokiol and vehicle for 48 h. Total RNA from RKO cells was extracted using Trizol (Invitrogen, USA). In RT-PCR, cDNA synthesis was performed using a RNA PCR kit (TaKaRA Biomedicals, Osaka, Japan) with the supplied oligo dT primer (Table 1). Reverse transcription was performed using a thermal program of 92 °C for 2 min, then 38 cycles of annealing for 30 s at 55 °C, extension for 90 s at 72 °C. As an internal control, semiquantitative analysis of β -actin was also amplified. This primer pair of β -actin had an optimal annealing temperature of 55 °C with 20 cycles and yielded a 350-bp PCR product. Samples were separated on 20 g/L agarose gel and visualized with ethidium bromide staining under UV light.
| Apoptosis modulator | Upstream primer (5’-3’) | Downstream primer (5’-3’) | Size (bp) |
| Bax | ACCAAGAAGCTGAGCGAGTGT | ACAAACATGGTCACGGTCTGC | 332 |
| Bcl-xl | GGAGCTGGTGGTTGACTTTCT | CCGGAAGAGTTCATTCACTAC | 379 |
| Bid | GAC CCG GTG CCT CAG GA | ATG GTC ACG GTC TGC CA | 586 |
| Bcl-2 | TTGTGGCCTTCTTTGAGTTCG | TACTGCTTTAGTGAACCTTTT | 332 |
| β -actin | AGGCCAACCGCGAGAAGATGACC | GAAGTCCAGGGCGACGTAGCAC | 350 |
RKO cells (5 × 106) treated with 5, 10 µg/mL honokiol and vehicle respectively for 24 h were lysed by 4 g/L trypsin containing 0.2 g/L EDTA, then collected after washed twice with phosphate-buffered saline (PBS, pH 7.4). Total protein extract from RKO cells was prepared using cell lysis buffer [150 mmol/L NaCl, 0.5 mol/L Tris-HCl (pH 7.2), 0.25 mol/L EDTA (pH 8.0), 10 g/L Triton X-100, 50 mL/L glycerol, 12.5 g/L SDS]. The extract (30 µg) was electrophoresed on 12 g/L SDS-PAGE and electroblotted onto polyvinylidene difluoride membrane (PVDF, Millipore Corp., Bedford, MA) for 2 h in a buffer containing 25 mmol/L Tris-HCl (pH 8.3), 192 mmol/L glycine and 200 mL/L methanol. The blots were blocked with 50 g/L nonfat milk in TBST washing buffer for 2 h at room temperature and then incubated at 4 °C overnight with antibodies. All antibodies were diluted in TBST according to the manufacturer’s instructions. After washed at room temperature with washing buffer, the blots were labeled with peroxidase-conjugated secondary antibodies.
RKO cells, SW480 and LS180 cells (1 × 104 in 100 µL) were seeded on 96-well plates in triplicate respectively. Following a 24-h culture at 37 °C, the medium was replaced with fresh medium containing vehicle control or various concentrations of honokiol in a final volume of 200 µL. Cells were incubated at 37 °C for 68 h. Then 50 µL of MTT (2 mg/mL in PBS) was added to each well, incubated for an additional 4 h, the plate was centrifuged at 1 000 r/min for 10 min, then the medium was removed. The MTT formazan precipitate was dissolved in 100 µL DMSO, shaken mechanically for 10 min and then read immediately at 570 nm by a plate reader (Opsys MR, Denex Technology, USA).
Statistical significance was determined by Student’s t-test. A P value of 0.05 or less was considered significant.
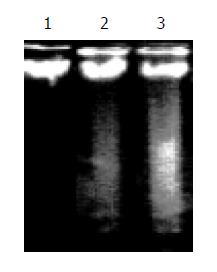
In honokiol-treated RKO cells, a degradation of chromosomal DNA into small internucleosomal fragments was evidenced by the formation of 180-200 bp DNA ladder on agarose gels (Figure 2), hallmark of cells undergoing apoptosis. No DNA ladders were detected in the samples isolated from control cultures. These results indicated that honokiol induced an apoptotic cell death in RKO cells
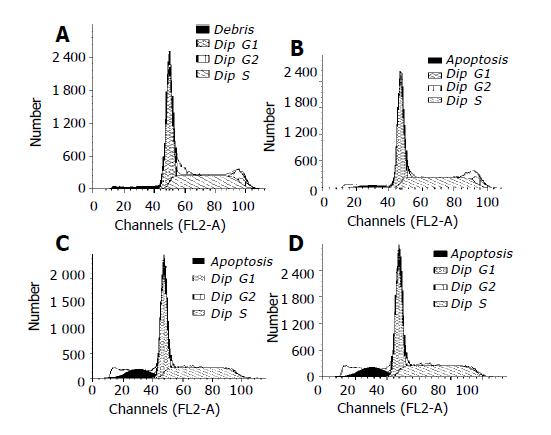
RKO cells were exposed to the increased concentrations of honokiol (5-15 µg/mL) for 48 h, and the cell growth was analyzed using flow cytometry. In the absence of honokiol, the cell populations were at G1, S, and G2/M phases (Figure 3), accompanied with increased concentrations of honokiol by a concomitant increase of the G1 phase (Table 2). From Figure 3, we considered the peak areas of subdiploid were up at the increased concentrations of honokiol. This observation led to a suggestion of G1 arrest. When the cells were exposed to honokiol at 10 µg/mL and above, subdiploid peak was significantly increased and the percentage of apoptotic cells in 10 000 cells was 14.1% and 20.31% respectively (Table 2).
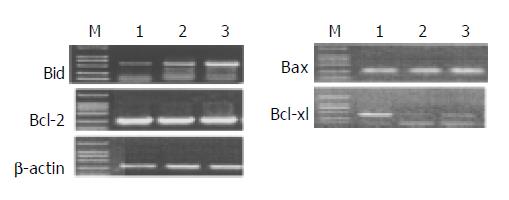
mRNA expression of apoptosis-related genes in response to different levels of honokiol was assessed. The Bcl-xl mRNA expression was significantly reduced in RKO cells exposed to honokiol, while Bid mRNA expression was remarkably up-regulated. No apparent changes of Bax and Bcl-2 were observed (Figure 4).
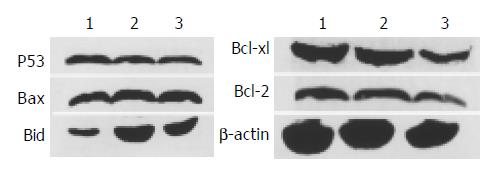
p53 and p53-related gene were found to be importantly involved in apoptosis induced by many agents. To study their role in honokiol-treated RKO, we monitored Bax, Bcl-2, Bcl-xl, Bid and p53 protein levels. Results in Figure 5 shows that no significant changes of Bax, Bcl-2 and p53 were found compared with vehicle. The level of Bcl-xl was decreased to vehicle level, while the level of Bid was increased remarkably after treated with 5 and 10 µg/mL honokiol respectively. The protein levels were measured by quantitative Western blot analysis after normalized with β -actin content.
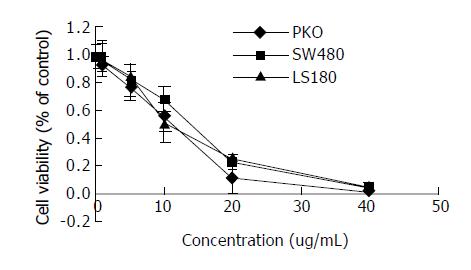
RKO cells had positively expressed of high wild-type p53 and SW480 cells had positive expression of high mutant p53, while LS180 had negative p53 antigen expression[20-22]. Cells treated with various concentrations of honokiol resulted in a dose- dependent cytotoxicity in three cells. As shown in Figure 6, honokiol-treated LS180 exhibited apparent growth inhibition like RKO cells and Sw480. Honokiol -mediated cytotoxicity occurred at the concentration of 5 µg/mL and above. A significant decrease in cell number was seen at 10 µg/mL. The concentration leading to a 50% decrease in cell number (IC50) was approximate 10.33, 12.98, 11.16 µg/mL in RKO, SW480 and LS180 cells respectively.
p53 is a crucial protein in cellular stress response. p53-dependent arrest of cells in the G1 phase of the cell cycle was an important component of the cellular response to stress[23]. Wild-type p53 was considered to participate in apoptosis in response to DNA damage in many tumor cells[14,24]. When cells received UV or ionizing radiation and were exposed to anticancer drugs, p53 protein was accumulated[25,26]. The increased p53 could transactivate its downstream target genes to induce cell cycle arrest, DNA repair, and apoptosis[27,28]. p53-dependent apoptosis was also observed in vivo[29]. However, p53-independent apoptotic cascades after administration of anticancer drugs or r-irradiation have been described.
In the present study, honokiol-treated RKO cells could induce apoptosis in a concentration- dependent manner, with the ratio of G1 phase increased. The agent also caused an increase of the expression of caspase-3 and caspase-7 in a dose-dependent manner, did not cause the significant increase of caspase-9 (data not shown). In CH27 cells it promoted to release cytochrome C and activate caspase 3[9].
p53-dependent apoptosis was activated by the Bax/ mitochondrial/caspase-9 pathway. Bcl-2 and Bax expression were regulated by p53 both in vitro and in vivo, and Bax was a direct target of p53 transcriptional activation[30]. Bax, a proapoptotic member of the Bcl-2 family, was located in the outer membrane of mitochondria[25]. Increased in the ratio of Bax/Bcl-2 could cause changes in the membrane potential of mitochondria, consequently, cytochrome C and other polypeptides were released from the intermembrane space of mitochondria into cytoplasm. Once released, cytochrome C could activate procaspase-9 by self-cleavage[12,13] and then other caspases[12].
According to previous data, honokiol led to cell G1 arrest and cytochrome C release like p53. Thus it is necessary to evaluate the mRNA and protein expression of Bax and Bcl-2. Our results showed that honokiol-induced apoptosis of RKO cells was accompanied with up- regulation of Bid and down-modulation of Bcl-xl, but did not involve the regulation of Bcl-2 or Bax protein expression. Since pro-apoptotic Bax is a p53 downstream target, the lack of change of Bax and Bcl-xl supports the view that honokiol probably does not trigger the p53/Bax-mediated apoptosis pathway. Next we examined the expression of p53 in honokiol-treated RKO cells. We found that no significant change of p53 was observed (Figure 5). Finally we analyzed honokiol-induced apoptosis of the three cells with different p53 expression. Our data showed that honokiol-treated LS180 and SW480 cells exhibited apparent growth inhibition like RKO cells.
These results suggest that honokiol-induced caspase activation and cell apoptosis are entirely controlled by the p53-independent pathway.
Co-first-authors: Tao Wang and Fei Chen
Edited by Wang XL Proofread by Chen WW and Xu FM
| 1. | Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol. 2003;475:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Teng CM, Chen CC, Ko FN, Lee LG, Huang TF, Chen YP, Hsu HY. Two antiplatelet agents from Magnolia officinalis. Thromb Res. 1988;50:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Liou KT, Lin SM, Huang SS, Chih CL, Tsai SK. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003;69:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Watanabe K, Watanabe H, Goto Y, Yamaguchi M, Yamamoto N, Hagino K. Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: central depressant effects. Planta Med. 1983;49:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukemic HL-60 cells. Life Sci. 1994;55:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 213] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Hibasami H, Achiwa Y, Katsuzaki H, Imai K, Yoshioka K, Nakanishi K, Ishii Y, Hasegawa M, Komiya T. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int J Mol Med. 1998;2:671-673. [PubMed] |
| 8. | Nagase H, Ikeda K, Sakai Y. Inhibitory effect of magnolol and honokiol from Magnolia obovata on human fibrosarcoma HT-1080. Invasiveness in vitro. Planta Med. 2001;67:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Yang SE, Hsieh MT, Tsai TH, Hsu SL. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem Pharmacol. 2002;63:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805. [PubMed] |
| 11. | Miyashita T, Harigai M, Hanada M, Reed JC. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131-3135. [PubMed] |
| 12. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5483] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 13. | Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM, Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359-8362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1034] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 15. | Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54:2095-2097. [PubMed] |
| 16. | Beham A, Marin MC, Fernandez A, Herrmann J, Brisbay S, Tari AM, Lopez-Berestein G, Lozano G, Sarkiss M, McDonnell TJ. Bcl-2 inhibits p53 nuclear import following DNA damage. Oncogene. 1997;15:2767-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Marin MC, Hsu B, Meyn RE, Donehower LA, el-Naggar AK, McDonnell TJ. Evidence that p53 and bcl-2 are regulators of a common cell death pathway important for in vivo lymphomagenesis. Oncogene. 1994;9:3107-3112. [PubMed] |
| 18. | Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805. [PubMed] |
| 19. | Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791-1798. [PubMed] |
| 20. | Smith ML, Chen IT, Zhan Q, O'Connor PM, Fornace AJ. Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene. 1995;10:1053-1059. [PubMed] |
| 21. | Rodrigues NR, Rowan A, Smith ME, Kerr IB, Bodmer WF, Gannon JV, Lane DP. p53 mutations in colorectal cancer. Proc Natl Acad Sci U S A. 1990;87:7555-7559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 712] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 22. | Xu LH, Deng CS, Zhu YQ, Liu SQ, Liu DZ. Synergistic antitumor effect of TRAIL and doxorubicin on colon cancer cell line SW480. World J Gastroenterol. 2003;9:1241-1245. [PubMed] |
| 23. | Luu Y, Bush J, Cheung KJ, Li G. The p53 stabilizing compound CP-31398 induces apoptosis by activating the intrinsic Bax/mitochondrial/caspase-9 pathway. Exp Cell Res. 2002;276:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337-7342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 452] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Li G, Bush JA, Ho VC. p53-dependent apoptosis in melanoma cells after treatment with camptothecin. J Invest Dermatol. 2000;114:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Li G, Ho VC, Mitchell DL, Trotter MJ, Tron VA. Differentiation-dependent p53 regulation of nucleotide excision repair in keratinocytes. Am J Pathol. 1997;150:1457-1464. [PubMed] |
| 27. | Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6842] [Cited by in RCA: 6816] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 28. | Li G, Ho VC. p53-dependent DNA repair and apoptosis respond differently to high- and low-dose ultraviolet radiation. Br J Dermatol. 1998;139:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Tron VA, Trotter MJ, Tang L, Krajewska M, Reed JC, Ho VC, Li G. p53-regulated apoptosis is differentiation dependent in ultraviolet B-irradiated mouse keratinocytes. Am J Pathol. 1998;153:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |