MATERIALS AND METHODS
Materials
We performed HIFU treatments in 100 patients (80 male, 20 female, ranging 30-74 years with mean age of 56 years) with liver cancer from July 2001 to July 2003, totally 130 HIFU treatments, 1.30 times per patient. Patients included 62 primary liver cancers and 38 metastatic liver cancers. Sixty-eight patients were with single nodule, 22 with two nodules, 10 with three nodules. Totally 36 tumor nodules with a diameter less than 5 cm, 76 with a diameter between 5-10 cm, 30 with a diameter more than 10 cm were involved. All cases were investigated and verified by pathohistology or an obvious increase of serum AFP and positive imaging, and conformed to diagnostic standard of National Cooperation Conference and Hepatic Carcinoma Prevention and Treatment in 1997. Eighteen patients were in stage I, 48 patients in stage II, and 34 patients in stage III.
Instrument
JC type focused ultrasound tumor therapeutic system was designed by Chongqing HIFU Technology Co, Ltd. Chongqing, China. It includes two main parts, i.e. ultrasound real-time orientation monitor device and HIFU three- dimensional combination scanning therapeutic device. Under the control of a computer, it can orient to preassigned tumor target zone automatically, determine range of therapy.
The main parameters included therapy frequency 0.8 MHz, mean diameter of focal field 1.1 mm, length of focal field 9.8 mm, focus distance 135 mm, therapy power 140-240 W.
Methods
Routine examinations and preoperative preparation were conducted according to the principle of surgery. Based on the result of image and ultrasound examination, the therapeutic scheme of HIFU was constituted. HIFU treatment was performed with patient fixed properly. The tumor position and size, therapeutic layers and therapeutic range of every layer were determined by ultrasound diagnostic probe. Then the therapeutic probe treated tumor tissue of every layer from outside body, and in terms of order of layer, from spot to line, and from line to area, leading whole tumor to coagulation necrosis. During the therapeutic process, through changes of graphics of target field and echo of tissue between before and after therapy at every layer, real-time estimate of HIFU therapeutic effect by computer processing image system was carried out, and with feedback, ultrasound therapeutic dosage estimated in the therapy scheme was controlled according to changes of ultrasound photograph. The therapeutic method was divided into complete coverage and local coverage. Twenty-eight cases used complete coverage, including whole tumor focus and normal liver tissue within 2 cm away from edge of tumor, the other cases used local coverage due to reasons, such as large tumor volume, rib overlap or close-by, or involvement of liver tube or cholecyst, etc.
Observatory parameters
The following aspects were observed: Improvement of clinic symptoms; changes of liver function 2 d and 2 wk before and after therapy; changes of AFP 2 wk before and after therapy; changes in range and blood supply of coagulation necrosis of tumor focus, also shrink of tumor through re-examination of MRI or CT before and after therapy.
RESULTS
One hundred liver cancer patients were treated by using HIFU in this group, of which 82 exhibited clinic symptoms. Seventy-one patients were improved obviously after HIFU treatment: appetite increased, weight gained, discomfort or pain on liver region relieved. Remission rate for symptoms was 86.6%(71/82). For 6 patients who had had mild ascites, ascites disappeared after HIFU treatment. For patients with abnormal liver function (ALT 95 ± 44 U/L, AST 114 ± 58 U/L), 2 d after HIFU treatment, liver enzymes rose slightly, but no obvious statistic difference was found compared with pre -treatment. The patients’ liver enzymes fell to normal level after 2 wk, respectively, ALT 83.3%(30/36), AST 72.9%(35/48). For the patients whose AFP was increased, AFP was 50% less than original level 2 wk after HIFU treatment, in 65.3%(32/49), only one patient’s AFP rose continuously after HIFU treatment, and multi-bone metastases were found by ECT examination. Self-comparison of MRI showed that T1-weighted images and T2-weighted images of tumor therapeutic region were high signal and low signal respectively after HIFU treatment. Enhanced MRI did not show enhanced signal, indicating that coagulation necrosis had occurred in the tumor therapeutic region, blood supply of tumor was diminished or eliminated, tumor of some patients had shrunk obviously after countercheck MRI (Figures 1-3).
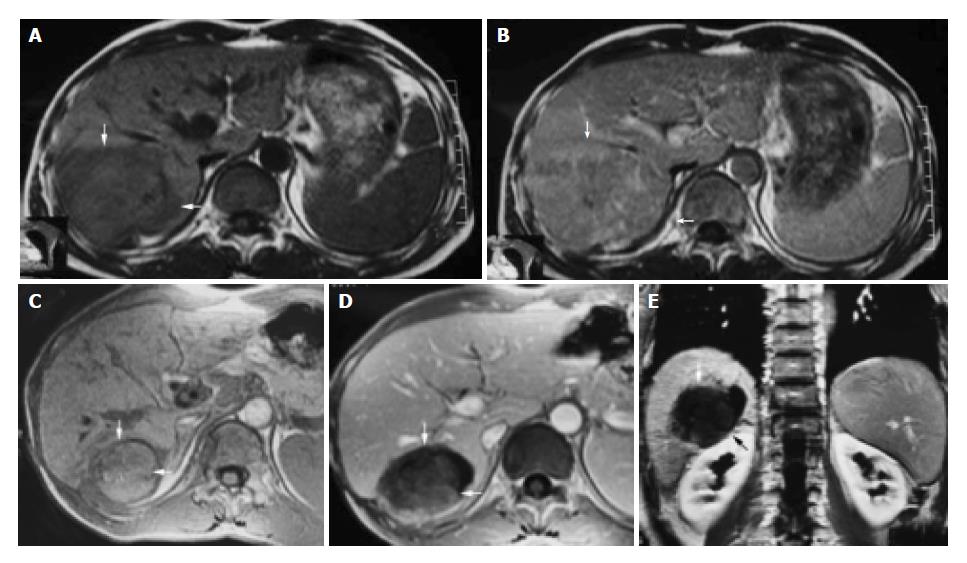
Figure 1 A 42-year-old male with hepatocellular carcinoma.
A: MRI T1-weighted images before HIFU treatment showed that liver tumor of right-posterior lobe was 115 mm × 100 mm × 66 mm; B: MRI enhanced imaging before HIFU revealed that enhancement of mass in liver right-posterior lobe was higher than that of surrounding normal liver tissues; C: MRI T1-weighted imaging 11 mo after HIFU revealed that liver tumor of right-posterior lobe obviously decreased in size (50 mm × 55 mm × 60 mm); D, E: MRI enhanced imaging (cross section and coronal section) 11 mo after HIFU revealed that liver tumor had shrunk obviously, blood supply of tumor was eliminated, the tumor therapeutic region had coagulation necrosis.
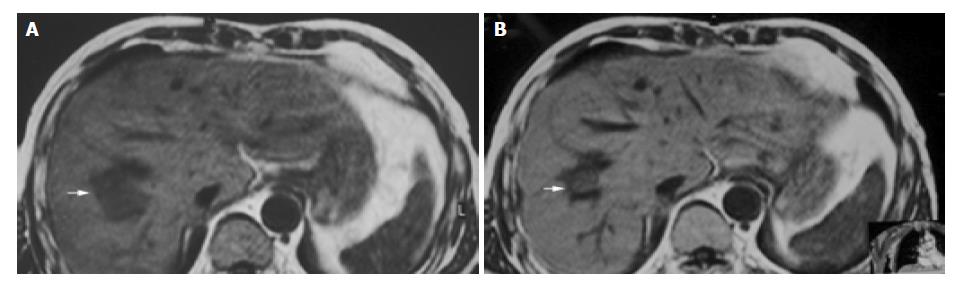
Figure 2 A 55-year-old man with metastatic hepatocarcinoma in sigmoid cancer postoperation.
A: MRI images before HIFU treatment showed that liver tumor of right-posterior lobe was 40 mm × 30 mm × 30 mm; B: MRI images 2 wk after HIFU revealed that liver tumor of right-posterior lobe obviously decreased in size (20 mm × 20 mm × 15 mm).
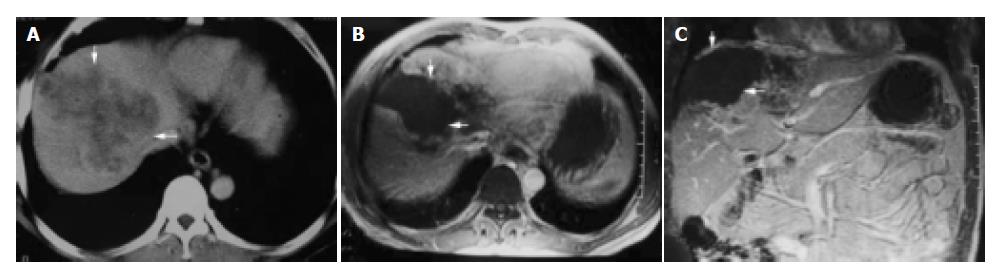
Figure 3 A 54-year-old man with primary carcinoma of liver in right lobe.
A: CT enhanced imaging before HIFU treatment revealed that enhancement of mass in liver right-posterior lobe was higher than that of surrounding normal liver tissues (AFP: 5516 μg/L); B, C: MRI enhanced imaging (cross section and coronal section) 2 mo after HIFU revealed that necrotic tissue in the therapeutic region of the tumor was not enhanced (AFP: 183 μg/L).
DISCUSSION
HIFU is a high-tech developed successfully in the 1990’s, a local way of treating tumor without any damages. It utilizes the physics characteristics of ultrasound beam with assemble and penetration, to focus low energy outside body on inner tumor target field, through instantaneous high temperature effect, cavitate effect, making tumor target tissue of focal zone coagulation necrosis, without destroying surrounding tissues[7-14].
HIFU has the following characteristics in therapy of malignant tumors. Firstly, noninvasiveness. HIFU treats inner tumor without damaging outside body. Previous research on animal angiography indicated that after treating liver tumor of Morris rat using HIFU, nourishing blood vessels which diameter was less than 200 µm of irradiation zone closed, but they were normal for blood vessels which diameter was more than 200 µm[15]. Secondly, accuracy. HIFU can pass through tissues and accurately damage target tissues inside organisms. The boundary between therapy zone and un-therapy zone is clear, tissue beyond target zone is hardly destroyed or without damage[16,17]. Ultrasound Therapy Section of London Emperor Hospital, Britain found that only six cells existed between cells killed completely and cells without damage[18]. Thirdly, real-time therapy. For whole process of therapy, it is a real-time targeting and monitoring process, real -time estimating therapeutic effect and adjusting dosage[19]. Fourthly, suitable therapy. According to the size and shape of tumor, it determines therapy range of tumor target zone, overlays tumor target zone. Therefore, treatment of malignant tumor using HIFU has many advantages such as less pain, no damage, fewer influences on splanchnic function, faster recovery for body and no increase of tumor metastasis chance[20], etc.
It is shown that in this research, 100 liver cancer patients had obvious improvement in symptoms and signs after being treated by using HIFU. Short-term effect of HIFU therapy was obvious and affirmative in liver cancer. But for liver cancer patients who had huge block or multifocal big nodules, to make tumor completely occur coagulation necrosis, to gain the purpose of “ablation” tumor integrity, we performed treatment for two or three times. Firstly, we treated deeper tumor parts, to induce coagulation necrosis of the tumor, so as to make it easy to treat remaining superficial parts next time. Conversely, if at first, it makes superficial parts occur coagulation necrosis, and then treats deep tumor tissue, because of change of the impedance dispersion and absorb coefficient of sound, the attenuation of penetrating tissue with ultrasound will aggravate. Meanwhile, focus energy in focal field can not reach ideal degree, as a result it takes more time to make tumor tissue coagulation necrosis.
If tumor locates on the edge of liver with poor blood supply, during therapy of HIFU, We estimate every therapeutic effect by using real-time ultrasound imaging to monitor the change of gray value. If gray value is changed, it indicates that tumor tissue must occur coagulation necrosis. However, tissues with coagulation necrosis do not always exhibit changes of gray value. For tumor in deep part, during real-time monitor, most changes of gray value are not distinct or lightly increased with suffusion. Through quantitative analysis and comparison of gray value of ultrasound imaging before and after therapy, we can find that the difference of gray rank before and after therapy is obvious. This indicates that local tumor tissues have no reversible coagulation necrosis.
For liver cancer patients with abundant blood supply relatively, we should first perform transcatheter arterial chemoembolization before using HIFU, so as to make inner tumor tissue have more iodised oil deposition, which not only is convenient to ultrasound orientation, but also changes the impedance dispersion and absorb coefficient of sound of tumor zone. Accordingly, it is convenient to energy sediment of focal zone, exerts cooperative function of raising temperature, excites high temperature to get to purpose of destruct therapy at local and makes tumor tissue coagulation necrosis[21,22].
Yang et al[15] used HIFU to treat Morris rat hepatoma implanted in the liver. Treatment was administered with a lens-focused 4- MHz transducer that created a focused beam of 550 W/cm2 at peak intensity. One hundred and twelve rats with liver tumors were divided into two groups of 56 each. Group 1 received HIFU therapy while group 2 (the control group) did not. All rats were killed immediately or 1, 3, 7, 14, 21, 28 d after treatment. Eight rats in each group were killed at each interval for pathologic and biochemical studies. Significant inhibition of the tumor growth was seen in the HIFU-treated group, with tumor growth inhibition rates of 65.4-93.1% from on d 3 to 28 after treatment. Ultrasound -treated tumors showed direct thermal cytotoxic necrosis and fibrosis. An additional 56 ACl rats with liver tumors were divided into 4 groups of 14 each. Group 1 received doxorubicin hydrochloride intraperitoneally and HIFU therapy; group 2, HIFU therapy; group 3, doxorubicin hydrochloride; and group 4 (the control group), neither HIFU nor doxorubicin hydrochloride. Significantly improved survival rates were noted in HIFU-treated animals (groups 1 and 2) compared with those of groups 3 and 4.
Research results of home and abroad using animals showed that HIFU can safely and effectively destroy inner liver tissue or liver transplant tumor[23-30]. Clinic results showed that HIFU can also treat liver cancer securely and effectively[11,31,32].
HIFU is a new method with definite therapeutic effect on tumor without damage and poisonous to normal tissues. With improvement of HIFU technology, accumulation of clinic application experience and further research on mechanism of HIFU, HIFU, as a new local therapy, would be applied more extensively to treat liver cancer.