INTRODUCTION
Liver cancer is one of the most common neoplasms worldwide. In some parts of Asia and Africa the prevalence is more than 100/100000 population[1]. Treatments often come to failure because of its high resistance to chemotherapy or radiotherapy and the severe side -effects induced[1]. At present the only curative options are partial hepaectomy or total hepaectomy with liver transplantation. But the chance for surgery is very limited. The effects of some other treatments such as intratumoral injection of ethanol or acetic acid, transarterial catheter embolization, and thermal destruction or microwave coagulation were also very poor due to the high incidence of tumor recurrence and hepatic failure[1-3]. Now combined treatments including biotherapy have been regarded as the most promising methods to cure liver cancer[4,5].
Among the biotherapies, antiangiogenic therapies have recently attracted an intense interest for their broad-spectrum action, low toxicity, and in direct endothelial targeting, the absence of drug resistance[6]. Antiangiogenic therapy induced by endostatin could specifically inhibit endothelial proliferation and potently inhibit angiogenesis and tumor growth accompanied by apoptosis in tumor cells[7-9]. Repeated cycles of antiangiogenic therapy were even followed by prolonged tumor dormancy without further therapy[10].
More and more learners have begun to realize that the occurrence of tumors is a complex course induced by multiple genes and multiple steps. Interference of single factor on tumor often can not get satisfactory results. Although primary tumors were regressed to dormant microscopic lesions after endostatin therapy, tumor cells were not eradicated at all[7,10]. Conventional chemotherapy or radiotherapy can do little on residual tumor cells, accurate recognition and clearance of which depends on the immune system of host[11]. IL-12 is among the most potent cytokines in stimulating antitumor immunity[12-14], which also showed significant inhibitory activity on angiogenesis[15].
So antiangiogenesis therapy by endosatin in combination with antitumor immunotherapy by IL-12 has become an attractive approach. On one hand synergistically antiangiogenic effect may be achieved to induce tumor dormancy. On the other hand, residual tumor cells may be also eradiated by antitumor immunotherapy induced by IL-12. Due to the problems such as bioactive protein production in large quantities, high costs and daily administration of endostatin[10,16], and the severe side-effects of system administration of IL-12[17,18], local gene therapy has proved to be very effective and nontoxic at the same time[19-24]. In this study combined intratumoral gene therapy of endostatin and IL-12 was observed in treatment of mouse hepatoma.
MATERIALS AND METHODS
Plasmid and host
Mouse endostatin eukaryote expression plasmid pSecES was constructed in our laboratory (data not shown). The expression of endostatin was under the control of cytomegalovirus (CMV) promoter. A mouse Igκ signal peptide sequence was located upstream of endostatin sequence to lead endostatin to secrete out. Mouse IL-12 eukaryote expression plasmid (pmIL-12) was a gift from Professor David M. Mahvi in Wisconsin University, USA. Both Mr 35 000 light chain gene and Mr 40 000 heavy chain gene linked by an internal ribsome entrance site were put downstream of CMV promoter[25]. Empty plasmid pcDNA3.1, pcDNA3.1-lacZ and E.coli DH5α were from the collection of our laboratory. DH5α bacteria containing the plasmids were grown in LB medium to mid-log phase. The plasmids were then purified with Qiagen columns (Qiagen). An analytical gel of each plasmid (cut and uncut) was done to ensure that there was no contamination with other nucleic acids. The A260/A280 ratios ranged from 1.8 to 2.0. Also each plasmid was confirmed to be free of endotoxin. For gene therapy, concentrated plasmid stock solution was made by lyophilizing and rehydrating with water. Then it was formulated into a solution containing 1 g/L plasmid, 50 g/L polyvinylpyrrolidone (PVP, K30, Mr 40 000, from Sigma), 150 mmol/L NaCl before use.
Cell culture and animals
BHK-21, human umbilical vein endothelial cell line (HUVEC) ECV304 and mouse hepatoma cell line H22 were purchased from China Center for Type Culture Collection. All the cell lines were maintained in RPMI 1640 medium (GibcoBRL) supplemented with 100 mL/L fetal bovine serum (FBS), 105 U/L penicillin and 100 mg/L streptomycin. Cells were cultured at 37 °C with 50 mL/L CO2. Six to 8-wk-old male BALB/c mice of specifical-pathogeny-free (SPF) grade (purchased from Hubei Experimental Animals Center, China) were maintained in a standard SPF animal’s room. All animal studies were performed in accordance with acceptable animal use guidelines.
Secretion and activity of endostatin plasmid and IL-12 plasmid
In 6-well plate, 5 × 105 BHK-21 cells per well were transfected with 1 µg pSecES or 1 µg pmIL-12, 3 µL LipoGen (InvivoGen) in accordance with directions of the manufacturer. Forty-eight hours after transfection, 0.1 mL ECV304 cells (2 × 107/L) cultured in RPMI 1640 additionally supplemented with 2 µg/L recombinant human basic fibro growth factor (rhbFGF) (PeproTech, UK) was inoculated to a 96- well plate. 0.1 mL supernant of BHK-21 cells transfected with pSecES was added to ECV304 cells per well. Seventy-two hours later, 20 µL 5 g/L MTT was added per well. After 4 h, the supernant was discarded and 150 µL DMSO was added. Absorbency at 570 nm was assayed. Forty -eight hours after transfection, mouse spleen cells were also produced by routine protocols and cultured in RPMI 1640 medium supplemented with 100 mL/L FBS for 2 h. Then the suspending lymphocytes were adjusted to 1 × 108/L and seeded to a 24-well plate (0.5 mL per well). Also 0.5 mL supernant of BHK-21 cells transfected with pmIL-12 was added per well. Forty-eight hours later, the proliferation was evaluated by MTT assay as above. The supernant of BHK-21 transfected with pcDNA3.1-lacZ was used as control. Also transfection rate was evaluated by X- gal staining of BHK-21 transfected with pcDNA3.1-lacZ 48 h later. All the three kinds of supernant was stored at –20 °C for further assay.
Combined gene therapy of endostatin and IL-12 for H22 hepatoma in vivo
In 0.1 mL PBS, 105 H22 cells were inoculated i.m. into the hind limb of BALB/c mice. Then the mice were randomly divided into five groups (6 per group): PVP/NaCl (untreated), pcDNA3.1 (empty vector), pSecES, pmIL-12, pSecES + pmIL-12. Another 6 mice were not inoculated for further assay of basic serum endostatin. From the next day after inoculation, 100 µg pSecES/PVP and/or pmIL-12/PVP, 100 µg pcDNA3.1/PVP or 100 µL PVP/NaCl solution was injected into the inoculation site respectively with a 26 G needle every three days. After inoculation for 21 days, tumors were removed and weighed. Before tumor dissection, mouse blood was collected and stored at 4 °C overnight. Then the serum was stored at –20 °C for further assay after centrifugation. The data were analyzed statistically by SPSS 10.0 procedure.
Assay of mouse endostatin and IL-12 (p70) in supernant or serum
The frozen supernant of BHK-21 transfected with pSecES, pmIL-12 or pcDNA3.1-lacZ and the frozen serum were thrawed to room temperature. Then mouse endostatin and IL-12 (p70) were assayed according to the directions of CytElisa mouse endostatin kit (CytImmune, USA) and mouse IL-12 (p70) ELISA kit (Shengzhen Jingmei Biotech, China).
Detection of MVD, TILs and apoptosis of H22 hepatoma
Paraffin sections (5 µm) of tumor tissues were stained with rat anti-mouse CD31 antibody (eBioscience). Then the primary antibody was detected by biotinylated secondary rabbit anti-rat IgG and SABC immunohistochemistry kit (Wuhan Boster Biotech, China) according to directions of its manufacturer. Paraffin sections were also stained with HE to reveal tumor infiltrating lymphocytes (TILs). Apoptosis of tumors was displayed by TdT-mediated dUTP nick end labeling (TUNEL) staining with an in situ cell death detection kit (Boehringer Mannheim, Germany) in accordance with the guardline of its manufacturer.
Tumor microvessel density (MVD) was counted as previously described[26,27]. The CD31 staining sections were scanned in 40 × microscope and “hot spots” (area with greatest vascula) were found, from which 3 random fields of 200 × microscope were selected. The average vascular number of the three fields was looked as MVD. For HE and TUNEL staining sections, 5 random nonnecrotic tissue fields in 400 × microscope was selected. The average number of TILs or apoptotic cells was counted. The data were analyzed statistically by SPSS 10.0 procedure.
RESULTS
Secretion and activity of endostatin plasmid and IL-12 plasmid
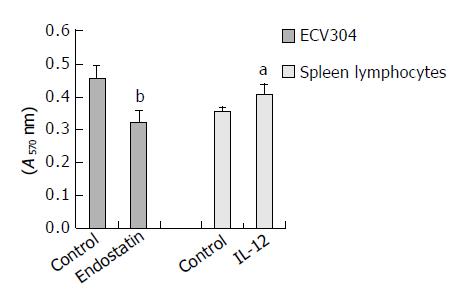
β -galactosidase expression was assessed by X-gal staining 48 h post-transfection. The tansfection efficiency was about 30%. No endostatin or IL-12 was detected in the supernant of BHK-21 transfected with pcDNA3.1-lacZ. Endostatin secreted by BHK-21 transfected with pSecES was 65.5 ± 10.9 ng/106 cells. When this supernant was added to ECV304 stimulated by rhbFGF, the proliferation of the latter was inhibited by 29.2% 72 h later (inhibitory efficiency = 1-A of endostatin group/A of control group, P < 0.01). Also 221.8 ± 44.4 ng/106 cells IL-12 (p70) was detected in the supernant of BHK-21 transfected with pmIL-12. It could promote mouse spleen lymphocytes to proliferate by 15.2% (proliferation efficiency = A of IL-12 group /A of control group-1, P < 0.05). The results are displayed in Figure 1.
Figure 1 Proliferation of ECV304 inhibited by the supernant of BHK-21 transfected with pSecES and proliferation of spleen lymphocytes stimulated by the supernant of BHK-21 trans-fected with pmIL-12.
Each bar represents A value of ECV304 or spleen lymphocytes, and mean ± SD for 5 wells. aP < 0.05 vs control, bP < 0.01 vs control.
Antitumor effect of combined gene therapy of endostatin and IL-12 for H22 hepatoma in vivo
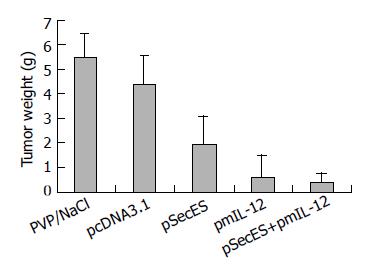
On the next day after inoculation, the mice received gene therapy. No skin lesions were found in the injection sites. On d 21 after inoculation, tumors were dissected and weighed. Big tumors could be found in all mice of PVP/NS group and pcDNA3.1 group. In pSecES group, all mice formed tumors, some of which were much smaller. Four mice formed tumors in pmIL-12 group and only two mice formed small tumors in pSecES + pmIL-12 group. The average tumor weight of pSecES group was much lower than that of PVP/NS group or pcDNA3.1 group (P < 0.01) (Figure 2). The inhibitory rate was 56.4% (inhibitory efficiency = 1-tumor weight of pSecES group/tumor weight of pcDNA3.1 group). For pmIL-12 group, the inhibitory rate was 85.7%. When pSecES gene therapy and pmIL-12 gene therapy were used in combination, tumor growth was inhibited by 91.8% (P < 0.01).
Figure 2 Hepatoma formation and growth inhibited by gene therapy of endostatin and IL- 12.
Each bar represents tumor weight of mice treated with PVP/NaCl, pcDNA3.1/PVP, pSecES/PVP, pmIL-12/PVP or pSecES + pmIL-12/PVP, and mean ± SD for six mice. P < 0.01 by ANOVA analysis.
Serum endostatin and IL-12 levels after gene therapy
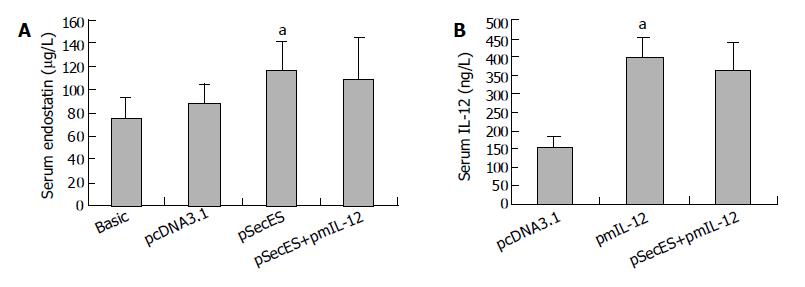
When gene therapy was finished, the serum endostatin and IL-12 levels were evaluated by ELISA. The serum endostatin level in pSecES group was 117.1 ± 12.8 μg/L, which was much higher than that in pcDNA3.1 group (87.7 ± 6.1 μg/L) or the basic level (75.8 ± 6.6 μg/L) (P < 0.05). Also, after pmIL-12 gene therapy, the serum IL-12 level was higher in pmIL-12 group (401.0 ± 51.6 ng/L) than in pcDNA3.1 group (154.8 ± 25.8 ng/L) (P < 0.05) (Figure 3).
Figure 3 Increase of mice serum endostatin levels or IL-12 levels after gene therapy.
A: Serum endostatin levels of mice treated with pcDNA3.1/PVP, pSecES/PVP, pSecES + pmIL-12/PVP or not inoculated with H22 cells (basic levels). Each bar represents mean ± SD for six mice. aP < 0.05 vs pcDNA3.1 group. B: Serum IL-12 levels of mice treated with pcDNA3.1/PVP, pmIL-12/PVP, pSecES + pmIL-12/PVP. Each bar represents mean ± SD for six mice. aP < 0.05 vs pcDNA3.1 group.
MVD, TILs and apoptosis of H22 hepatoma after gene therapy
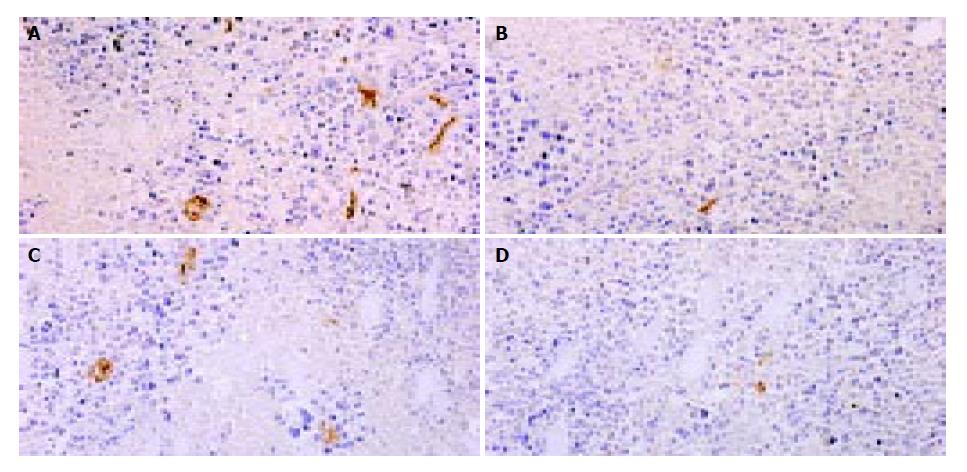
MVD were counted after CD31 staining in tumor sections. Compared with pcDNA3.1 group, MVD of hepatoma treated with pSecES/PVP or pmIL-12/PVP were much less. And in pSecES + pmIL-12 group, MVD were less than any single gene therapy group (P < 0.05) (Figure 4).
Figure 4 Hepatoma angiogenesis inhibited by gene therapy of endostatin and IL-12.
MVD (CD31staining, 200 × ) of hepatoma treated with pSecES/PVP (7.3 ± 1.2) (B), pmIL-12/PVP (10.0 ± 1.7) (C) or pSecES + pmIL-12/PVP (3.7 ± 1.2) (D) was less than that treated with pcDNA3.1/PVP (22.7 ± 3.1, P < 0.05) (A).
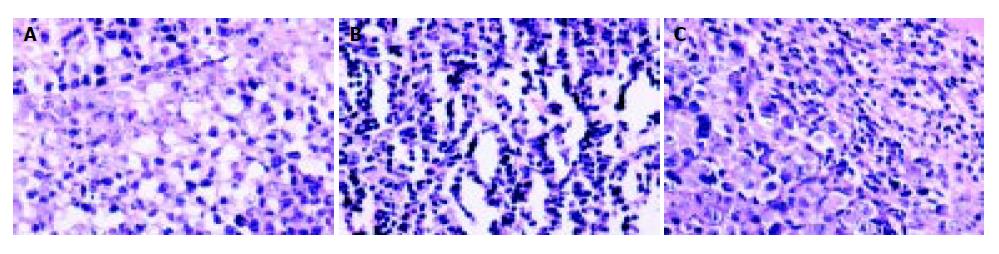
Tumor sections were stained by HE, then TILs were evaluated. After pmIL-12/PVP or pSecES + pmIL-12/PVP gene therapy, TILs in hepatoma were more than that treated with pcDNA3.1/PVP (P < 0.01). The results are displayed in Figure 5.
Figure 5 Lymphocytes infiltration into hepatoma promoted by gene therapy of IL-12.
TILs (HE staining, 400 × ) of hepatoma treated with pmIL-12/PVP (146.2 ± 24.6) (B) and pSecES + pmIL-12/PVP (123.2 ± 21.4) (C) were more than that treated with pcDNA3.1/PVP (45.2 ± 11.8, P < 0.01) (A).
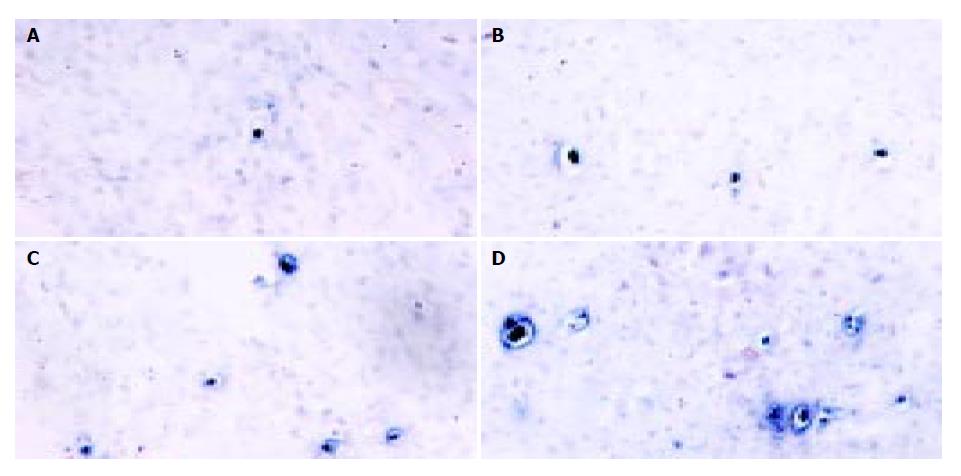
Also, tumor apoptosis was displayed by TUNEL staining. Figure 6 shows that there were only a few apoptotic tumor cells dispersed in 400 × random scopes in pcDNA3.1 group. But in pSecES group or pmIL-12 group, apoptotic tumor cells increased. In pSecES + pmIL-12 group, apoptotic tumor cells were much more than any group (P < 0.05).
Figure 6 Apoptosis of hepatoma cells induced by gene therapy of endostatin and IL-12.
Apaptotic tumor cells (TUNEL, 400 × ) treated with pSecES/PVP (11.2 ± 2.3) (B), pmIL-12/PVP (14.4 ± 3.5) (C) or pSecES + pmIL-12/PVP (24.8 ± 4.8) (D) were more than those treated with pcDNA3.1/PVP (1.4 ± 1.7, P < 0.05) (A).
DISCUSSION
Despite the progress in early diagnosis of liver cancer, its prognosis remains poor. In average, the mean life span was less than a year after diagnosis [1]. How to prevent or cure the recurrence of remained tumor cells after partial hepaectomy and how to inhibit tumor infiltrating in liver cancer patients in advanced stage are urgent challenges for liver cancer therapy. Now antiangiogenic therapy and immunotherapy have been paid more and more attention and many promising conclusions have been drawn[28-31]. But in clinical trails, few satisfactory results were obtained by single biotherapy[17,18,32]. Whether synergistic effects of antiangiogenesis therapy in combination with immunotherapy could be achieved is worthy to be researched.
Studies showed that angiogenesis played a very important role in the course of growth and metastasis of solid tumors including liver cancer. When the tumor size was beyond 2 mm3-3 mm3, oxygen and nutrition were not enough by simple diffusion. Then new vessels must be formed for further growth[33]. However, angiogenesis was dependent on the cooperation of pro-angiogenesis factors and anti -angiogenesis factors[34]. Among many inhibitors, endostatin, the C terminal of collagen X VIII, which is a Mr 20 000 protein with 184 amino acids in primary structure, has the strongest antiangiogenesis and antitumor effect. It was identified from the medium of a murine hemangioendothelioma (EOMA) cell line in 1997[7]. Researchers indicated that both natural and recombinant endostatin could markedly inhibit endothelial cell proliferation in vitro and angiogenesis in chick chorioallantoic membrane, but had no effect on non-endothelial cells. When endostatin was administrated subcutaneously, primary tumors were regressed to dormant microscopic lesions and metastasis was inhibited. Repeated cycles of endostatin therapy showed prolonged tumor dormancy without further therapy[7,10]. In our previous study, subcutaneous injection of 10 mg/(kg.d) endostatin produced by gene engineering showed a potent inhibitory effect on the formation and growth of mouse H22 hepatoma[35]. Also the clinical trail of endostatin has been carried out[32]. But to obtain both high quantity and high quality of endostatin was necessary for optimal therapeutic benefit[36]. It was indicated that the activity of endostatin expressed in mammalian cells was 1 000 times than recombinant protein extracted from E.coli[37]. So gene therapy of endostatin has become a very good solution and many studies suggested that gene therapy of endostatin showed a powerful inhibition on liver cancer[21,22].
When H22 hepatoma cells, derived from Balb/c mouse liver cancer in 1940 s, were inoculated into the leg muscle of mice, solid tumors formed quickly, which were easy to touch and easy for injection. Compared with viral vectors, non-viral vectors bore some advantages such as easy to handle, high capacity for DNA sequences, low toxicity, and non-immunogenic thus permitting repeated vector administrations[38]. So we constructed endostatin eukarytic plasmid pSecES, inside which a mouse Igκ signal peptide sequence was put upstream endostatin sequence to let endostatin secrete out. In vitro high-levels endostatin with inhibitory activity on the proliferation of endothelial cells could be detected from the supernant after transfection. And in vivo, for lower degradation of input DNA by endosomes and/or lysosomes and higher transfection efficiency, non-toxic PVP was used to enhance gene expression according to previous reports[39,40]. Plasmid/PVP was injected intratumorally and repeatedly for a higher local concentration. After endostatin gene therapy, mice serum endostatin levels were consistently 2-3-fold higher than basic levels. Compared with controls, tumors formed slowly with less MVD and more apoptosis cells. The average tumor weight inhibitory rate was 56.4%. It was indicated that pro-angiogenic factors such as VEGF were induced when tumor vessels formed gradually, and antiangiogenic factors such as endostatin increased for a negative regulation[41,42]. This opinion was also confirmed by our study that serum endostatin levels in mice burdened with big tumors after vector injection were increased compared with basic levels. When more endostatin were added by gene therapy, the balance would be broken and angiogenensis was inhibited.
Immunocytokines which were important in the regulation of the immune system could overcome immune tolerance against tumor antigens, thus tumor rejection was facilitated. It was benefic for prevention of recurrence of micro tumor cells and advanced tumors which could not accept operation or chemotherapy[11,13]. Different cytokines (IL-2, IL-4, IL-12, INF-γ , TNF-α , GM-CSF) have been used to modulate the host’s immune response either in vitro or in vivo. The activation and proliferation of immunocytes and antitumor immunity were induced more or less[13]. IL-12 is among the most potent cytokines in stimulating antitumor immunity. It is a disulfide-linked heterodimeric cytokine. IL-12 acts by inducting a TH1 type of response, activating natural killer cells and cytotoxic T lymphocytes, enhancing expression of adhesion molecules on endothelial cells, thus facilitating the traffic of lymphocytes to the tumor, and inducting a potent antiangiogenic effect. In previous researches, the antitumor effect of IL -12 was established in various in vivo systems[12-14]. However, the latter was reported to cause fatal toxicity in a clinical trial[17], which led investigators to local administration of this cytokine by gene therapy or gene gun, that proved to be very effective and nontoxic at the same time[23,24]. In our study, active IL-12 (p70) was expressed in vitro and in vivo. Tumors did not formed in 30% mice that received IL-12 gene therapy. The formed tumors were also highly inhibited with more TILs, less MVD and more apoptotic tumor cells. Moreover, the inhibitory effect of IL-12 gene therapy on H22 hepatoma was much better than that of endostatin plasmid (P < 0.05).
When combined gene therapy of endostatin and IL-12 was used, its antitumor effect was much better than others. Tumor formation was not observed in 66.7% mice. The average tumor weight inhibitory rate was 91.8%. But in mice that received endostatin gene therapy, no tumor regressed. The average tumor weight inhibitory rate was 56.4%. No tumor was formed in 33.3% mice after they received IL-12 gene therapy. The average tumor weight inhibitory rate was 85.7%. It was suggested that synergistic antitumor effect was achieved after combined gene therapy of endostatin and IL-12. The proposed mechanism of the combined gene therapy maybe a synergistic cooperation of an antiangiogenesis effect induced by endostatin and IL-12 in combination with an immune rejection induced by IL-12. On one hand, endostatin and IL-12, which were expressed locally in combination with increased serum levels after gene delivery mediated by plasmid with PVP, could inhibit angiogenesis of hepatoma synergisticly. This was convinced by that MVD in pSecES + pmIL- 12 group was much less than any single gene therapy group. But now the antiangiogensis mechanism of endostatin and IL-12 was still not very clear. Through this way, H22 hepatoma cells came to apoptosis or necrosis due to lack of blood supply. On the other hand, more TILs (including activated CTL, NK, TH1 etc.) were observed in tumors that received IL-12 gene therapy or combined gene therapy, which maybe an indication of activated immune response on H22 hepatoma cells. Antitumor immune rejection induced by IL-12 through immunocytes or cytokines also promoted apoptosis of tumor cells. So there were much more apoptotic tumor cells in combined gene therapy group than any single one. Due to these factors, formation and growth of transplanted H22 hepatoma was prevented synergisticly.
Combined gene therapies for liver cancer were also reported previously. Learners have focused on combined genes of two or three immunocytokines, or on suicide gene therapy in combination with genetic immunotherapy[25,30,31,43]. Biotherapy in combination with chemotherapy for lymphoma was also studied[44]. Before our reports, combined treatment with type 5 adenovirus vectors expressing murine angiostatin and IL-12 showed an antitumor effect on a murine breast cancer[45]. Our report is the first to demostrate the potent antitumor effect of combined gene therapy of endostatin and interleukin 12 with polyvinylpyrrolidone on hepatoma.
In conclusion, active target protein can be expressed by repeated local injections of plasmid with PVP. Through this way, a short- term course of combined gene therapy of endostatin and IL-12 can effectively prevent the formation and development of transplanted H22 hepatoma in mice. Antiangiogenic therapy in combination with immunotherapy may be used to regress or to eradicate other solid tumors.