Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2067
Revised: February 8, 2004
Accepted: February 28, 2004
Published online: July 15, 2004
AIM: To confirm the xenotransplantation of microencapsulated hepatocytes and islets as a temporary bioartificial liver support system for mice with acute liver failure (ALF).
METHODS: Mice were rendered ALF by a single intra-peritoneal injection of D-galactosamine (D-gal) and their tail blood was sampled to examine differences in blood ALT, albumin (ALB), total bilirubin (TB) and glucose (GLU) between 4 experimental groups. Rat hepatocytes and islets were collected and microencapsulated referring to both Sun’s and Fritschy’s methods. Mice were grouped into control group (CG), free hepatocyte group (FHG), microencapsulated hepatocyte group (MHG) and microencapsulated hepatocyte plus islet group (HIG). Tissue samples were subjected to microscopic and electron microscopic (EM) examinations.
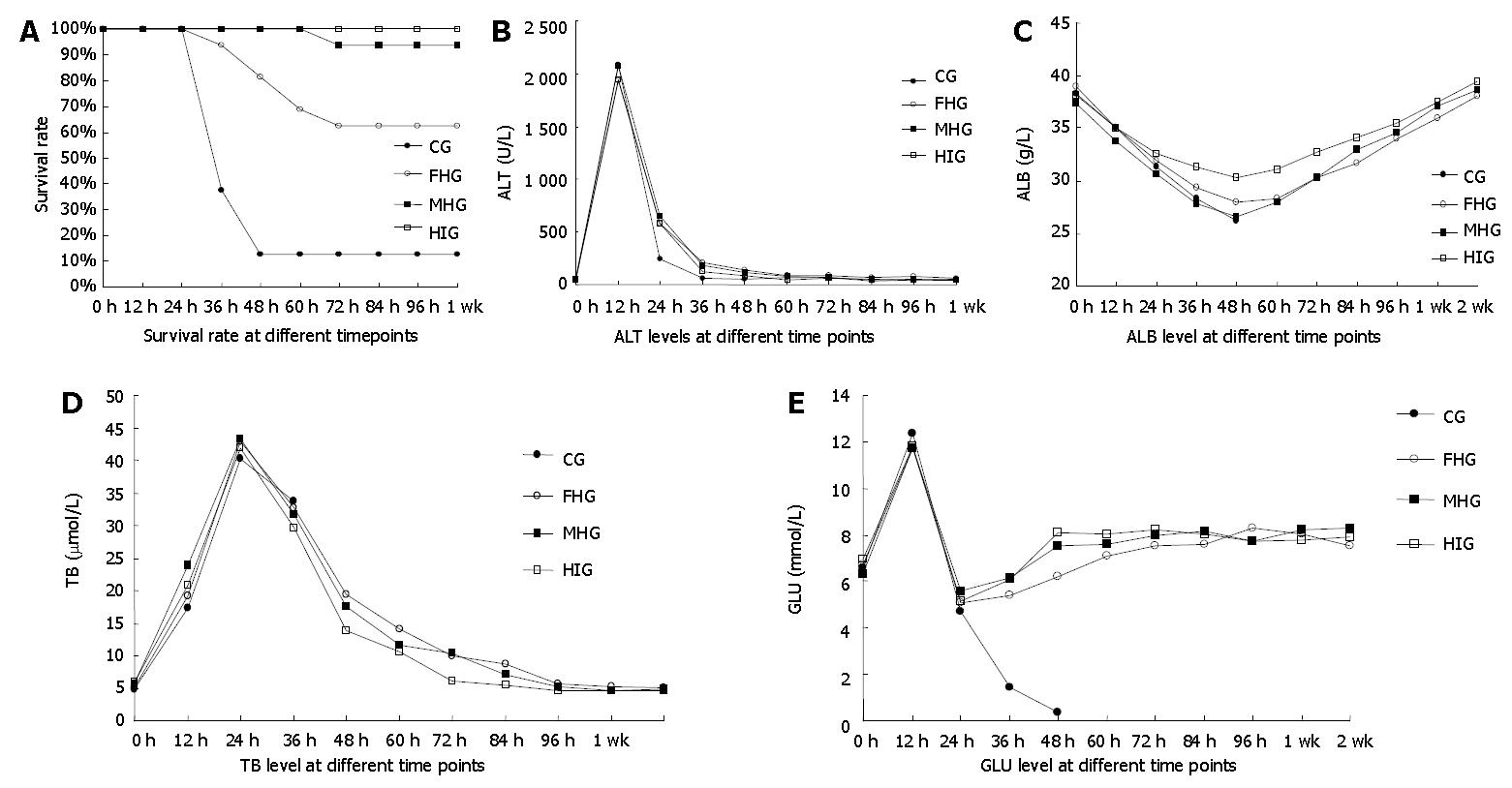
RESULTS: The highest survival was observed in HIG, surprisingly at 100% (16/16), while the lowest was in CG at 12.5% (2/16), with inter-group statistical difference P < 0.05. ALT levels revealed no statistical difference between groups but the ALB level of HIG descended by the slightest margin {q = (0.54, 0.24, 1.33), P < 0.05} at the time when it reached the lowest point in all groups. TB of HIG returned to normal reference range (NRR) statistically sooner than that of others after a fierce elevation. No statistical inter-group difference was observed in GLU levels. Fusion between hepatocytes and beta cells was demonstrated giving rise to theoretical assumptions.
CONCLUSION: Hepatocytes to be microencapsulated together with islets should be a preferred in vivo hepatic functional supporting system, which can dramatically prolong survival and improve living status.
-
Citation: Gao Y, Xu J, Sun B, Jiang HC. Microencapsulated hepatocytes and islets as
in vivo bioartificial liver support system. World J Gastroenterol 2004; 10(14): 2067-2071 - URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2067
Currently, temporary liver support is crucial to medical treatment for acute liver failure[1,2]. In this point, hepatocyte transplantation as a kind of bioartificial liver holds a bright prospect, but how to enhance graft functional status and neutralize immune rejection remains unsettled. Several authors have reported the “mutual benefit” between hepatocytes and pancreatic islets at co-transplantation[3-5], whereas Lim and Sun’s[6] microencapsulating technique for immunoisolation opened a new era for cell transplantation. We suppose that hepatocytes and islets can be co-microencapsulated and transplanted to act as a bioartificial liver support system (BALSS), thus taking advantages of both the “mutual benefit” and the immunoisolation, simultaneously. If succeeded, this method would be a valuable alternative for en bloc organ transplantation and alleviate the donor shortage dilemma.
Male Wistar rats weighing 150 g (as donor of hepatocytes) or 250 g (as donor of islets) and Kunming mice weighing 30-36 g served as donors and recipients, respectively. D-gal (Sigma) was dissolved in bacteriostatic normal saline and adjusted to pH7.4 with 1 mol/L NaOH. The final concentration of the solution was 0.1 g/mL. Acute liver failure (ALF) mice was induced by a single intra-peritoneal injection of D-gal at a dose of 2500 mg/kg without anesthesia, after which free access to aseptic water containing 100 g/L glucose was provided and models were fed on standard mouse chow.
Donor hepatocytes were isolated by Seglen’s in situ collagenase perfusion technique. Under pentobarbital anesthesia (35 mg/kg), the portal vein and the before-liver inferior vena cava (IVC) were cannulated and after liver IVC was ligated. The liver was perfused in situ first with 4 °C D-Hanks solution for 3 min and then with 37 °C Hanks solution containing 200 U/mL collagenase IV (Sigma) for 10 min at a flow rate of 20 mL/min until the effluent turned opaque. The perfusion route began at the portal vein and ended before liver IVC. The liver was removed, softly shattered and filtered through a hepatocyte dispersing instrument (containing a 105 µm stainless steel mesh) to produce a homogenous suspension. The hepatocytes were washed 3 times in 4 °C D-Hanks solution and centrifuged at 50 r/min for 2 min between washes, (3.5-5.0) × 108 hepatocytes were obtained through this process. After the cell viability (82%-93%) was determined by trypan blue exclusion test, the hepatocytes were cultured in 100 g/L fetal calf serum (FCS, Hyclone) enriched RPMI-1640 medium containing 10-7 mol/L insulin, 0.1 mg/mL streptomycin sulfate and 100 U/mL penicillin G at 37 °C in a humidified atmosphere (950 mL/L O2 and 50 mL/L) awaiting transplantation.
Donor islets of Langerhans were extracted by Sutton’s method. The common bile duct (CBD) was cannulated and the rat was killed by a heart incision before the whole pancreas was distended uniformly with 4 mL 4 °C Hanks solution (buffered by 20 mmol/L HEPES at pH7.8). The pancreas was removed, further distended by 16 mL 4 °C Hanks solution containing 2 mg/mL collagenase V (Sigma) and then put in an incubator at 38 °C for 8 min before the tissue was dispersed. The pelleted tissue was filtered through a steel mesh with 40 pores per inch and washed twice in 4 °C Hanks solution with centrifugation at 50 r/min for 1 min between washes. The last centrifugation was performed at 350 r/min for 2 min before the tissue was re-suspended in 25% Ficoll solution, on which 23%, 20% and 11% Ficoll densities were layered in sequence. The discontinuous density gradient containing the tissue pellets was spun at 800 r/min for 15 min at 4 °C. Islets from 20%-23% interface were collected, 721 ± 153 islets could be isolated through this procedure with a purity higher than 87% and the ones with a diameter of 50-250 μm were handpicked and cultured in 100 g/L FCS enriched RPMI 1640 medium containing 0.1 mg/mL streptomycin sulfate and 100 U/mL penicillin G at 37 °C in a humidified atmosphere (950 g/L O2 and 50 mL/L CO2).
Cells were microencapsulated according to Sun’s and Fritschy’s methods. The cells were centrifuged before re-suspension in 30 g/L alginate (ALG)-normal saline solution (pH7.4) at a concentration of 1 × 107/mL (according to hepatocytes). Then the mixture was put into the container of a bi-nozzle air-jet droplet generator and sprayed into 100 mmol/L calcium chloride solution (HEPES buffered, pH7.4) under the disturbance of a co-axial oxygen flow at 4 L/min to form round droplets, which were then washed in 4 °C D-Hanks solution and reacted in sequence with 2 g/L poly-L-lysine (PLL) for 8 min, 2 g/L ALG for 4 min and 30 mmol/L sodium citrate for 8 min, all were kept at 4 °C and adjusted to pH7.4. The microcapsules (MCs) with a diameter of 0.3-0.5 mm were cultured in aforementioned medium for 24 h. Every mouse received MCs equal to 3 × 106 hepatocytes and the ratio of hepatocytes to islets was 106/40 at co-transplantation[7]. Through a 12 G needle, the MCs were injected into the recipient’s abdomen through a 3 mm incision under light ether anesthesia. All mice underwent the same procedure.
Sixty-four mice with ALF were randomly divided into 4 groups, 16 mice in each: CG, FHG, MHG and HIG. Each mouse in a certain group received only 1 mL sterilized normal saline in CG, 3 × 106 free hepatocytes transplantation in FHG, 3 × 106 microencapsulated hepatocytes in MHG, MCs containing 3 × 106 hepatocytes together with 120 islets in HIG.
Each group was randomly divided into 4 subgroups, 4 mice in each: ALT group, ALB group, TB group and GLU group so that every index was observed in 4 mice and documented by their average. Blood samples were collected from tail veins and indexes were recorded before and every 12 h after administration of D-gal (time “0”) until the 4th d, then once on the 7th d and finally once on the 14th d. The body mass was measured daily.
All animals that died after D-gal injection were autopsied and those alive were sacrificed weekly until the 2nd mo. Then all the remaining ones were cervically dislocated to collect samples from livers, MC aggregates and floating MCs, which were fixed either in 100 g/L phosphate-buffered formaldehyde and stained with hematoxylin/eosin for histological examination or in 2.5% osmium tetroxide for transmission EM examination.
One-way ANOVA, q test and χ2 test were used to establish inter-group difference and data were expressed as mean ± SD.
Mice still alive after 72 h secured unlimited survival. Compared with CG, the survival rates of FHG, MHG and HIG were statistically higher (P < 0.05), while FHG held a survival rate significantly lower than MHG and HIG (P < 0.05), between which no statistical difference was observed (P > 0.05) (Figure 1A).
ALT in 4 groups similarly elevated to a peak of 1912 ± 344 U/L in 12 h, then dropped quickly to NRR (50.04 ± 7.72 U/L) in 48 to 72 h. The sub-group of ALT in CG had only one mouse (1/4) alive after 36 h. No relevant statistical difference was observed (Figure 1B).
Serum ALB gradually decreased after D-gal administration and reached the lowest point in 48 h, then stably returned to NRR of 38.19 ± 1.68 g/L in 96 h (Figure 1C). The maximum decrease in ALB of HIG was 7.85 ± 1.10 g/L, statistically smaller than that of other groups (P < 0.05). No statistical difference was found in ALB reduction between CG, FHG and MHG (P > 0.05).
TB in all groups ascended quickly after D-gal administration to the maximum of 36.64 ± 5.08 μmol/L (P > 0.05) in 24 h, then began to descend. All the 4 mice in TB sub-group of CG died in 36 h bringing its curve to a stop, while TB of other groups continued to descend to NRR (5.42 ± 0.68 μmol/L) at different intervals. TB of HIG fell into NRR in 72 h, while FHG and MHG in 96 h (Figure 1D).
The non-fasting blood glucose of ALF mice temporarily elevated by 5.27 ± 1.56 mmol/L in 12 h after D-gal administration, then fell down to 5.14 ± 0.48 mmol/L at the 24th hour, when the mice underwent scheduled treatments. After 24 h, when the GLU of other groups began to increase, that of CG kept decreasing until all the four responsible mice died. The GLU of HIG ascended to NRR (8.31 ± 0.93 mmol/L) in 48 to 60 h, while that of FHG and MHG normalized after 72 h (Figure 1E). No statistical inter-group difference was observed at any time point (P > 0.05).
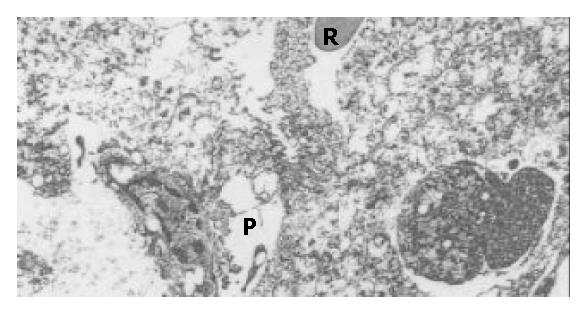
Massive degenerative changes, ballooning and necrosis of the hepatic cells with vanishing nuclei were apparent under optical microscopy accompanied with closing sinusoids, widened Disse space and distorted hepatic lobules in tissue samples from mice died after administration of D-gal. Numerous bulbs, slightly distended mitochondria, shrinkage of nuclei and pigmentation of heterochromatin came under EM with cholangitis, exfoliation of microvilli and disappearance of tight conjunctions between adjacent hepatocytes dominating the view, as reported by Takenaka[8] (Figure 2).
Up to 90% of microcapsules remained free floating in 1 to 2 wk posttransplantation. Histological examination revealed smooth surface of most MCs, but mild fibroblasts were also observed on some MCs. Few hepatocytes were still alive inside the MCs from MHG, which were still free floating in the 4th week. Fewer free-floating MCs were observed in the HIG, blood vessels originated from the portal vein or the liver surface were easily seen with naked eyes polarizing huge aggregates of MCs attached to them. Till the 8th week, red aggregates of MCs were easily found in the vicinity of the portal vein in 67.5% (7/8) mice from HIG group. Though not abundant, aggregates attached to the diaphragmatic side of the liver, spleen or other organs were also seen. Smaller aggregates of MCs were seen on samples from MHG but without visible vascularization.
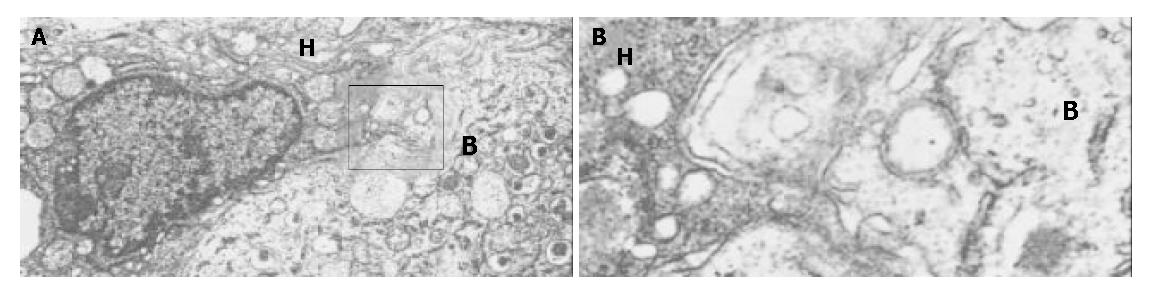
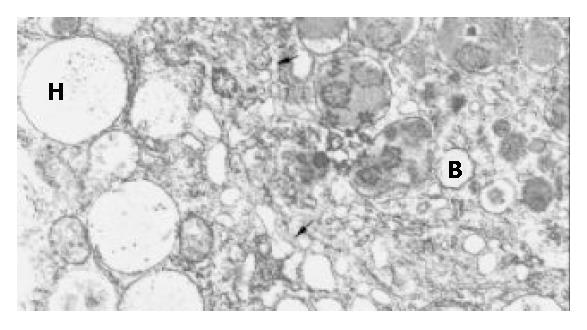
Generally, the wall of the MCs was 6-8 μm thick and could be divided into 3 layers, but hepatocytes were always embedded in it de-homogenizing the thickness and disarranging the layers. Mostly, a very thin feather-like structure with a medium density formed the outer layer, inside was a section with a very high density and a thickness of approximately 0.5 μm, then a flocculent low density structure formed the inner layer. There was no clear boundary between the later two layers, instead, the density of the middle layer lowered until it changed into the inner one, to which the encapsulated hepatocytes attached with their microvilli. A small number of hepatocytes of MHG group survived inside the MCs until the 8th week but their microvilli shortened or disappeared and mitochondria distended. The MCs from HIG attached to the liver surface were totally covered by fibroblasts in the 8th week, but capillaries originated from the liver were seen encircling the MCs or entering them. Inside these MCs, living beta cells were found surrounded by transformed hepatocytes, whose many microvilli thrust deeply into the former and the membranes of both cells morphologically vanished at some places where two plasmas mixed (Figure 3A, Figure 3B). Some hepatocyte-like cells recognized by their big round mitochondria and glycogen had well formed insulin granules inside their plasma but with a smaller size (Figure 4). No desmosome mentioned by Ricordi was found. Several ball-shaped hepatocytes with long microvilli and normal organella were seen attached to the MC wall in distance of the aggregates of hepatocytes and beta cells.
Life sustaining is of the greatest importance to patients with ALF during life threatening period and patients with end stage chronic liver failure (CLF) awaiting proper donors, so liver function support in a limited period is crucial to the management of both ALF and CLF.
Based on this principle, many kinds of artificial liver were developed such as charcoal hemoperfusion or high porosity hemodialysis, but optimistically speaking, these methods could only partially substitute for the original liver because besides functioning living hepatocytes, any man-made device could provide but an incomplete liver function support[9].
Artificial liver based on living hepatocytes has become a hot point of current researches aimed at substitution for liver function by hepatocytes. There are two kinds of BALSS currently in research: ex vivo bio-hemodialysis system and in vivo hepatocyte transplantation strategy. In recent years, great progress has been made in the ex vivo systems. Chen et al[10] demonstrated the successful application of BALSS to canine liver failure and Ding et al[11] reported their initial success on human patients. But, unfortunately, the viability of hepatocytes was hard to sustain extracorporeally and they gradually lost their original functions and de-differentiated in 1 to 2 wk in vitro, so that frequent refreshing of the working hepatocytes is necessary to secure satisfactory efficiency, which adds extra-cost to the BALSS besides its already expensive electromechanical system. As for hepatocyte transplantation strategy, rejection aggressively reduced hepatocytic viability to almost zero in 1 wk[12] after allo-transplantation. Great attention has been paid to the mechanism of hepatocytic rejection and a recent study showed that recombinant ribonuclease P remarkably reduced the expression of MHC-II molecules on transplanted hepatocytes, thus obviously alleviating rejection and prolonging hepatocytic survival. But before the entire truth of human genome is unveiled, no induction of gene or genetic products into human body would be ethically accepted and clinically approved. Genetic interference with transplantation may be preserved for far future. The microencapsulating technique initiated by Lim and Sun brought a bright new hope for artificial liver and hepatocyte transplantation. The microcapsules act as a selective barrier between the hepatocytes inside and the killing molecules such as antibodies outside, reserving the permeability of nutrients and small active cellular products such as albumin and insulin, so that cell transplantation under immunoisolation is realized. In addition, it has been proved that through some uncertain pathways islets could enhance the viability and function of isolated hepatocytes[13,14]. We deem that microencapsulated hepatocytes and islets can be xenotransplanted to act as an in vivo BALSS, thus taking advantages of both the “mutual benefit” and the immunoisolation simultaneously. If succeeded, this method would be a valuable alternative for en bloc organ transplantation and alleviate the donor shortage dilemma.
In this work, we initially studied the feasibility of xeno-transplantation of co-microencapsulated hepatocytes and islets between closely related species (Wistar rats to Kunming mice) and its therapeutic effects on D-gal induced ALF. Drug-induced ALF could better imitate clinical ALF, mostly toxic, than surgically induced ALF, since it presented with similar pathological development[15].
ALT is a sensitive index to hepatocytic destruction, which sharply ascended after administration of D-gal and peaked at the 12th hour but the amplitude greatly varied between individual mice. No significant influence upon ALT curve by cell transplant was observed in this study indicating that ALT could reflect the extent of cell destruction instead of the therapeutic effect of different interventions, as confirmed by Yu’s studies[16]. Anti-apoptotic agent etoposide was given prior to the administration of D-gal in Nakama’s study[17], which would possibly have changed the metabolism of D-gal in hepatocytes and the pathological process of hepatocytic destruction, preventing a considerable proportion of hepatocytes from apoptosis, so that a lowered ALT peak was documented. In fact, to give hepatocytic protective agents prior to the establishment of ALF model could only demonstrate the protecting effects of such agents instead of their therapeutic attributes, which has attracted much more attention from medical community since ALF patients would not take liver protective medications before they got trapped in ALF.
ALB is not a frequently used index to hepatic failure, but it changes with a steady pace giving concrete confirmation to the status of hepatic anabolism. In this study, ALB levels in HIG group decreased by a margin significantly smaller than those in other groups after administration of D-gal, indicating that under the influence of xenografted islets, at least the production of ALB of the remaining liver cells of the model mouse or the transplanted hepatocytes was improved. Wang et al[18] also confirmed the enhanced ALB synthesis in hepatocirrhotic rats after combined hepatocyte-islet transplantation.
TB level reflects the metabolic and detoxicating abilities of hepatocytes. It descended into NRR much earlier in HIG than in other groups, demonstrating a sooner recovery of hepatocellular metabolism in the existence of islets as stated by Wang et al[19].
Temporary increase of blood glucose after D-gal administration, was rarely reported because most researchers took rats as subjects and kept an interval between blood samplings as long as 24 h or more, missing this phenomenon. It might have resulted from the release of a great amount of glycogens at massive hepatocytic destruction, which was absent in studies adopting 90% partially hepatectomized models as in Demetriou’s[20]. Though curves in Figure 1E show a sooner extrication from hypoglycemia in HIG group than in MHG group, no statistical difference in GLU was noticed between experimental groups, which might have been the result of inadequate samples or large scale fluctuation of non-fasting blood glucose of ALF mice.
All mice in HIG group survived and the survival rate in MHG group was 93.75%, astonishingly higher than that in other reports, which used rat models. The first reason for such a high survival might be a larger amount of hepatocytes transplanted, say, 3 × 106 hepatocytes were nearly 3%-5% of total hepatocytes per mouse liver and the second reason might be a much shorter hepatocytic regeneration cycle in mouse, which resulted in a quicker recovery of liver function and a shorter life threatening period. In this point of view, it may be conjectured that the shorter the hepatocytic regeneration cycle is, the better the therapeutic effect of bio-artificial liver can be expected, thus to shorten the regeneration cycle of liver cells by means of some genetic method may further enhance the survival rate after ALF.
A close functional relation between hepatocytes and islets has been proved both experimentally and clinically. An “intimate association” between hepatocytes and beta cells was discovered by Ricordi[21] and then confirmed by others[22]. In this study, an even closer relationship was proved morphologically inside the microcapsules 2 mo after hepatocyte-islet co-transplantation.
Under EM, 1the hepatocytes were easily recognized with their wheel-shaped mitochondria, smooth reticulum, rose-petal shaped glycogens and Golgi complexes while beta cells with their homogeneous eyeball-like insulin granules, which accommodated high-density crystalloids at the center with a wide vacuum space around. The hepatocytes abandoned their original hexagon or cubic shape and flattened to wrap on the beta cells thrusting numerous finger-like microvilli deeply into the latter. No matter how complicatedly the microvilli wound in three-dimensional space on a cross-section, we should see two layers of complete membrane separating these “fingers” from the plasma of the victim beta cells, but discontinuous membranes were noticed where direct exchange of cellular content was suspected. Much more interestingly, the boundary between some hepatocytes and beta cells was replaced by a complicated network of plasmatic “bridges” formed by adjacent membranes of a string of vesicles lining up along the original border. Meanwhile morphologically intact beta granules were found inside such hepatocytes in direct contact with their plasma, seemingly having been swallowed. Generally, this was a kind of “hybridization” between these two kinds of cells, as mentioned by Cossel[23]. No desmosomes were found connecting these cells as mentioned by Ricordi. They might have functioned as anchors in the earlier stage (in 7 d as said by Ricordi) to immobilize cells giving rise to further interactions, then disappeared together with part of the membrane initiating hybridization in the later stage as found in our study. Direct plasmatic connection must be the best way for intercellular communication and exchange of substances, so contacting cells, which have close functional ties like hepatocytes and beta cells, would eventually establish such connections though neither the underlying mechanism nor its prognosis is clear. On the other hand, this phenomenon urges us to take good care of transplanted cells since it is still unknown whether such a “hybridization” produces pathogenic outcomes, say malignancies, or whether it undermines long-term effects of transplanted cells, though the outcome of such an association was fairly acceptable in our study. So that to separate transplanted cells by way of micro- or macro-encapsulation may be a good choice, which deprives them of direct contact with host cells and at the same time realizes functional support under immunoisolation.
In this study, we observed the hybridization between hepatocytes and beta cells inside microcapsules after co-transplantation. Better therapeutic effect of hepatocyte-islet co-transplantation than hepatocytes alone was also evidenced both histologically and biochemically. Though operative principles have not been fully established, co-transplantation of hepatocytes and islets by way of microencapsulation as a kind of BALSS holds a bright prospect.
We are gratefulness to Wei Liu, Center of Scientific Research, 2nd Hospital of Harbin Medical University for sharing her expertise in cell bioengineering and to Wen-Jie Dai, National Center of Experimental Cell Transplantation for his administrative help during this project.
Edited by Wang XL Proofread by Chen WW and Xu FM
| 1. | Fritschy WM, Wolters GH, van Schilfgaarde R. Effect of alginate-polylysine-alginate microencapsulation on in vitro insulin release from rat pancreatic islets. Diabetes. 1991;40:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Ricordi C, Callery MP, Lacy PE, Flye MW. Pancreatic islets enhance hepatocellular survival in combined hepatocyte-islet-cell transplantation. Transplant Proc. 1989;21:2689-2690. [PubMed] |
| 4. | Genin B, Andereggen E, Rubbia-Brandt L, Birraux J, Morel P, Le Coultre C. Improvement of the effect of hepatocyte isograft in the Gunn rat by cotransplantation of islets of Langerhans. J Pediatr Surg. 1999;34:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kaufmann PM, Sano K, Uyama S, Breuer CK, Organ GM, Schloo BL, Kluth D, Vacanti JP. Evaluation of methods of hepatotrophic stimulation in rat heterotopic hepatocyte transplantation using polymers. J Pediatr Surg. 1999;34:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1572] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 7. | Kaufmann PM, Kneser U, Fiegel HC, Pollok JM, Kluth D, Izbicki JR, Herbst H, Rogiers X. Is there an optimal concentration of cotransplanted islets of Langerhans for stimulation of hepatocytes in three dimensional matrices. Transplantation. 1999;68:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Takenaka K, Sakaida I, Yasunaga M, Okita K. Ultrastructural study of development of hepatic necrosis induced by TNF-alpha and D-galactosamine. Dig Dis Sci. 1998;43:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Dixit V, Darvasi R, Arthur M, Brezina M, Lewin K, Gitnick G. Restoration of liver function in Gunn rats without immunosuppression using transplanted microencapsulated hepatocytes. Hepatology. 1990;12:1342-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Chen XP, Xue YL, Li XJ, Zhang ZY, Li YL, Huang ZQ. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol. 2001;7:706-709. [PubMed] |
| 11. | Cai ZH, Shi ZQ, Sherman M, Sun AM. Development and evaluation of a system of microencapsulation of primary rat hepatocytes. Hepatology. 1989;10:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Zhang YD, Xu YM, Peng J. Viability and histological changes of encapsulated rat hepatocyte after transplantation. Zhonghua Qiguan Yizhi Zazhi. 2001;22:161-163. |
| 13. | Kaufmann PM, Sano K, Uyama S, Schloo B, Vacanti JP. Heterotopic hepatocyte transplantation using three-dimensional polymers: evaluation of the stimulatory effects by portacaval shunt or islet cell cotransplantation. Transplant Proc. 1994;26:3343-3345. [PubMed] |
| 14. | Kaufmann PM, Fiegel HC, Kneser U, Pollok JM, Kluth D, Rogiers X. Influence of pancreatic islets on growth and differentiation of hepatocytes in co-culture. Tissue Eng. 1999;5:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Papalois A, Arkadopoulos N, Papalois B, Pataryas T, Papadimitriou J, Golematis B. Comparison of two experimental models for combined hepatocyte-islet transplantation. Transplant Proc. 1994;26:3473. [PubMed] |
| 16. | Yu CH, Leng XS, Peng JR, Wei YH, Du RL. Morphology, structure and function of microencapsulated hepatocytes after intraperitoneal transplantation and its liver function support in acute hepatic failure rats. Zhonghua Shiyan Waike Zazhi. 1998;15:441-443. |
| 17. | Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, Ido A, Hayashi K, Tsubouchi H. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001;33:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Xue J, Zhang Z, Zhou Y. [The influence of intrahepatic transplantation of hepatocytes and insular cells on liver cirrhosis]. Zhonghua Waike Zazhi. 1998;36:179-181. [PubMed] |
| 19. | Wang XD, Ar'Rajab A, Ahrén B, Andersson R, Bengmark S. Improvement of the effects of intrasplenic transplantation of hepatocytes after 90% hepatectomy in the rat by cotransplantation with pancreatic islets. Transplantation. 1991;52:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier-attached hepatocytes into 90% partially hepatectomized rats. Hepatology. 1998;8:1006-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Ricordi C, Lacy PE, Callery MP, Park PW, Flye MW. Trophic factors from pancreatic islets in combined hepatocyte-islet allografts enhance hepatocellular survival. Surgery. 1989;105:218-223. [PubMed] |
| 22. | De Paepe ME, Keymeulen B, Pipeleers D, Klöppel G. Proliferation and hypertrophy of liver cells surrounding islet grafts in diabetic recipient rats. Hepatology. 1995;21:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Cossel L, Wohlrab F, Blech W, Hahn HJ. Morphological findings in the liver of diabetic rats after intraportal transplantation of neonatal isologous pancreatic islets. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |