Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2039
Revised: February 13, 2004
Accepted: February 26, 2004
Published online: July 15, 2004
AIM: To construct a DNA vaccine against extracellular domains 1-3 of fetal liver kinase-1 (flk-1), and to investigate its preventive and therapeutic effect against H22 cell in vivo.
METHODS: Flk-1 DNA vaccine was produced by cloning extracellular domains 1-3 of flk-1 and by inserting the cloned gene into pcDNA3.1 (+). Fifteen mice were divided into 3 groups and inoculated by vaccine, plasmid and saline respectively to detect specific T lymphocyte response. Thirty Mice were equally divided into preventive group and therapeutic group. Preventive group was further divided into V, P, and S subgroups, namely immunized by vaccine, pcDNA3.1 (+) and saline, respectively, and attacked by H22 cell. Therapeutical group was divided into 3 subgroups of V, P and S, and attacked by H22, then treated with vaccine, pcDNA3.1 (+) and saline, respectively. The tumor size, tumor weight, mice survival time and tumor latency period were compared within these groups. Furthermore, intratumoral microvessel density (MVD) was assessed by immunohistochemistry.
RESULTS: DNA vaccine pcDNA3.1 (+) flk-1-domains 1-3 was successfully constructed and could raise specific CTL activity. In the preventive group and therapeutic group, tumor latency period and survival time were significantly longer in vaccine subgroup than that in P and S subgroups (P < 0.05); the tumor size, weight and MVD were significantly less in vaccine subgroup than that in P and S subgroups (P < 0.05). The survival time of therapeutic vaccine subgroup was significantly shorter than that of preventive vaccine subgroup (P < 0.05); the tumor size, and MVD of therapeutic vaccine subgroup were significantly greater than that of preventive vaccine subgroup (P < 0.05).
CONCLUSION: DNA vaccine against flk-1 domains 1-3 can stimulate potent specific CTL activity; and has distinctive prophylactic effect on tumor H22; and also can inhibit the tumor growth in vivo. This vaccine may be used as an adjuvant therapy because it is less effective on detectable tumor.
-
Citation: Lü F, Qin ZY, Yang WB, Qi YX, Li YM. A DNA vaccine against extracellular domains 1-3 of flk-1 and its immune preventive and therapeutic effects against H22 tumor cell
in vivo . World J Gastroenterol 2004; 10(14): 2039-2044 - URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2039.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2039
Extensive studies by many investigators established that angiogenesis has a central role in the invasion, growth and metastasis of solid tumors[1-3]. The inhibition of tumor growth by attacking the tumor’s vascular supply offers a primary target for anti-angiogenic intervention. There are several advantages of targeting proliferating endothelial cells in the tumor vasculature rather than directly to tumor cells. First, endothelial cells are genetically stable and do not down regulate MHC-class I and II antigens. Second, the therapeutic target is tumor-independent. Thus, killing of proliferating endothelial cells in the tumor microenvironment can be effective against a variety of malignancies and clones[3,4]. Vascular endothelial growth factor (VEGF) plays a center role in tumor angiogenesis, while fetal liver kinase-1 (flk-1), which expressed exclusively on the endothelial cells, is the key receptor that binds VEGF[5,6]. Blocking the VEGF-flk-1 pathway may inhibit tumor angiogenesis. Hence, we constructed a DNA vaccine to target the extracellular domain 1-3 of flk-1 and tested its preventive and therapeutic effect against H22 cell in vivo with the hope that the vaccine might be able to inhibit angiogenesis via targeting flk-1 and flk-1 expressing endothelial cell. This research has no parallel reports at home.
Cell line Tumor cell H22 cell line, constructed by Dalian Medical University from the mouse ascite, was heterogeneous cells with higher declination to spread by lymph vessel. COS7 cell is derived from African green monkey kidney and preserved by our laboratory. Endothelial cells were primarily cultured from mouse vessel by our laboratory.
Animal BALB/c mice were bought from Laboratory Animal Center of the Fourth Military Medical University. All mice were male, specific pathogen free animals, with age of 6-8 wk and weight of 15-20 g.
Main reagents pcDNA3.1(+) was purchased from Invitrogen (USA). CD31 antibody was a product of Santa Cruz (USA). pMDT-18 Vector, T4 DNA ligase, TaqEX DNA polymerase, Hind III, EcroR I, and dNTP were products of TaKaRa (Japan). M-MLV reverse transcriptase was purchased from Promega (USA). E.Z.N.AR gel extraction kit was purchased from Omega Bio-tek (USA). Trizol, lipofectamine 2000, DMEM and G418 were purchased from Gibco (USA). Western blot luminescence kit was purchased from NEN (Britain). Micro BCA protein assay was purchased from Pierce (USA).
Culture of cells H22 cell was transferred in abdomen cavity of BALB/c mouse. The ascites were taken from the mouse in the super clean bench and then diluted with DMEM to 1 × 107/mL. A total of 0.2 mL of 1 × 107 H22 cells were inoculated subcutaneously at the right armpit of mouse to establish a tumor model. The interval between the ascites taking and the last mouse inoculation was less than 2 h. COS7 cell was cultured in DMEM containing 100 mL/L FCS and 50 mL /L CO2 at 37 °C. Primary culture of endothelial cells (ECs) were performed as follow: the mouse thoracic aorta was sterile dissociated, and sheared into small pieces about 0.5 cm × 0.5 cm, washed with Hank’s fluid, its EC surface was sticked to 1.5% gelatin culture medium, and then DMEM containing 100 mL/L FCS and benzylpenicillin (100 U/mL) and streptomycin (100 U/mL) was slowly added, then cultured at 37 °C under humidified atmosphere containing 50 mL/L CO2 until the cells form a single layer. Transfer of cell culture was performed (≤ 3 generations).
Construction of the expression vector encoding murine flk-1 domain 1-3 Total cellular RNA of mouse liver tissue was extracted using TRIZOL reagent and cDNA was prepared by random priming from 2 µg of total RNA using M-MLV according to the manufacturers’ instructions. PCR was performed for 35 cycles as follows: denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 80 s. Oligonucleotide primers to amplify flk-1 domain 1-3 transcripts were designed on the mouse sequence: upstream primer: 5’-CGGATAACCTGGCTGACC-3’; and downstream primer 5’-AGGGATTCGGACTTGACTGC-3’. Then the cloned gene was inserted into the pMDT-18 vector, followed by cut out from pMDT-18 by EcoR I and Hind III and insertion into the pcDNA3.1 vector between the restriction sites EcoR I and Hind III generating pcDNA3.1 (+)-flk-1-Domain1-3. Reading frame and sequence were proved right by DNA sequencing.
Expression of the pcDNA3.1 (+)-flk-1-domain 1-3 in the COS7 cell COS7 cells were plated in antibiotic free growth medium and cultured until about 90% confluence at the time of transfection. A 4 µg of pcDNA3.1 (+)-flk-1-Domain1-3 was diluted and mixed in 250 µL of DMEM, 10 µL of lipofectamineTM 2000 was diluted and mixed in 250 µL of DMEM respectively. They were mixed together after 5 min of incubation at room temperature, and incubated for 20 min then poured into 35 mm wells plated with COS7. Mixed gently and incubated at 37 °C in 50 mL/L CO2 incubator for 48 h. Cultured cells were incubated for 20 min and sonicated twice for 5 s in cold lysis buffer. Total extracts were cleared by centrifugation for 30 min at 4 °C at 14000 g. Protein levels were quantified with Micro BCA protein assay. Samples were resolved by 120 g/L SDS-polyacrylamide gel electrophoresis (SDS-PAGE). In all gels, 80 µg of protein dissolved in sample buffer was loaded per lane. Immunoblotting was performed by electroblotting onto nitrocellulose membrane (0.2 mm) at 32 mA for 1 h using a semidry immunoblotter. Membranes were blocked for 2 h at room temperature with blocking buffer and subsequently incubated for 1 h at room temperature with anti-flk-1 polyclonal antibody at a 1:200 dilution with blocking buffer. The enhanced chemiluminescence system was used for the detection of bound antibody. Primary antibody-antibody-bound membranes were incubated for 1 h at room temperature with horseradish peroxidase conjugated antirabbit or antimouse IgG at a 1:1000 dilution with blocking buffer. After washing with TBS and TBST, the membranes were treated with enhanced chemiluminescence reagents according to the manufacturer’s protocol. The membranes were exposed to X-ray film for 2 min.
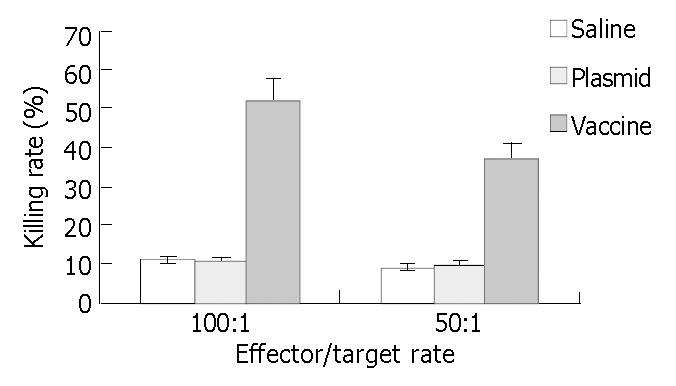
Analysis of specific cytolytic T lymphocyte (CTL) activity Fifteen mice were classified to three groups: vaccine group, plasmid group, and saline group, each group had 5 mice. Mice were injected with 100 µg of vaccine, 100 µg of plasmid, and 100 µg of saline respectively, at the concentration of 1 µg/µL once every 10 d. All the mice were killed on day 10 after the last immunization and the spleen lymphocytes were separated and cultured in complete culture medium with interleukin-2 (1 × 105 U/L) and 100 mL/L FCS at saturated humidified atmosphere containing 50 mL/L CO2 at 37 °C to induce the CTL. CTL and mouse ECs was mixed at 100:1 and 50:1 effector/target rate, respectively. In addition, there were a CTL control group, an ECs control group and a culture medium control group. The specimens of each group had 3 parallel wells on a 96-well culture plate and were cultured under conditions of saturated humidity at 37 °C containing 50 mL/L CO2 for 48 h. Cell-mediated cytotoxicity was determined by using a standard 51Cr release assay. Briefly, target cells were pelleted and resuspended in 3.7 GBq of 51Cr per 106 cells and incubated at 37 °C in a humidified 50 mL/L CO2 incubator for 1 h. They were washed three times with media, resuspended to 5 × 104/mL, and 0.1 mL was added to round-bottomed microtiter wells. Varying numbers of effector cells were added in 0.1-mL volume to achieve the desired E/T ratios. For a spontaneous 51Cr-release control, 0.1 mL of media was substituted for effector cells. Maximum release was determined by adding 0.1 mL of 1% NP-40 to the target cells. After 6 h at 37 °C, the plates were centrifuged at 1200 r /min for 5 min, and 0.1 mL of supernatant was taken from each well and counted on a gamma counter. Data are presented as percent specific 51Cr-release = 100 × [(experimental group counts per minute (cpm) - spontaneous group cpm)/(maximum release cpm - spontaneous cpm)].
Preventive effect of pcDNA3.1 (+)-flk-1-domain1-3 against H22 Thirty BALB/c mice were randomly divided into V, P, and S subgroups, 10 mice in each subgroup, and immunized by 100 µg vaccine, plasmid, and saline, respectively, at concentration of 100 µg /µL, once every 10 d by intramuscular injection at left hip. Five days after the last immunization, all mice were injected with 1 × 107 of H22 cells at the right armpit subcutaneously. The mice weight, tumor formation, tumor size and mice survival time were recorded. Tumor volume was measured in 2 dimensions and calculated as follows: length/2 × width2. After mice death, tumor tissue was routinely fixed and embedded in paraffin and then performed CD31 staining. All mice were kept observation until death.
Therapeutic effect of pcDNA3.1 (+)-flk-1-domain1-3 against H22 Thirty BALB/c mice were randomly divided into V, P, and S subgroups, ten mice in each subgroup. All mice were injected with 1 × 107 of H22 tumor cell subcutaneously at right armpit. Four days later, tumor was formed in all mice, which demonstrated the successful construction of tumor model. Mice in different groups were treated with 100 µg of vaccine, plasmid, and saline, respectively, at the concentration of 1 µg/µL, two times every 5 d by intramuscular injection at left hip. The tumor weight and size were recorded each day. The mice weight, tumor formation, tumor size and survival period of mice were recorded. After mice death, tumor tissue was routinely fixed and embedded in paraffin for CD31 staining. All mice were kept observation until death.
Counting of microvessel density in H22 solid tumor Tumor tissue was washed with cold PBS, then fixed in 40 g/L buffered fomaldehyde solution and embedded in paraffin. Tissue sections (4 μm thick) were preincubated for 30 min with PBS containing 3 mL/L hydrogen peroxide and then blocked with blocking solution containing 50 mL/L goat serum. CD3 staining was performed with a polyclonal rabbit anti-rat CD31 antibody diluted in PBS (1:50) supplemented with blocking solution for 24 h at 4 °C. Finally, sections were incubated with the biotinylated secondary antibody followed by streptavidin-horse-radish-peroxidase complex. Antibody binding was visualized with diaminobenzidine (DAB). Vessel counts within the tumor were assessed by light microscopy after CD31 staining. Based on the criteria of Weidner et al[7], a vessel lumen was not required for the identification of a microvessel.
The five areas with the highest number of discrete microvessels in each slide were identified by scanning tumor sections at low power. Then the number of microvessels were counted in these areas at 200 magnification (0.75 mm2 area) to obtain accurate microvessel density. The average value of the five areas was counted as MVD.
Results were expressed as mean ± SD. Data were analyzed by the method of ANOVA using SPSS 10.0 software. P < 0.05 was considered statistically significant.
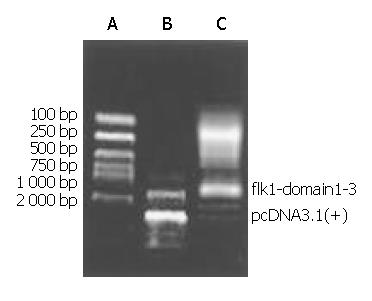
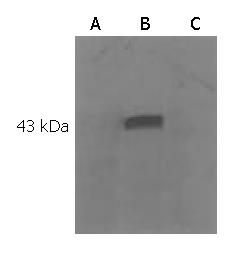
A 1250 bp gene part was cloned from mouse liver tissue using RT-PCR technique (Figure 1). DNA sequencing showed that pcDNA3.1 (+) flk-1-domain1-3 was the right reading frame and sequence. Western blotting of the lipotransfected COS7 cell showed that the expression of a Mr 44000 protein in the cell after transfection of the pcDNA3.1 (+)-flk-1-domain1-3 (Figure 2, Figure 3).
CTL activity of mice in vaccine subgroup was significantly higher than that of plasmid and saline subgroup (Figure 4, P < 0.05).
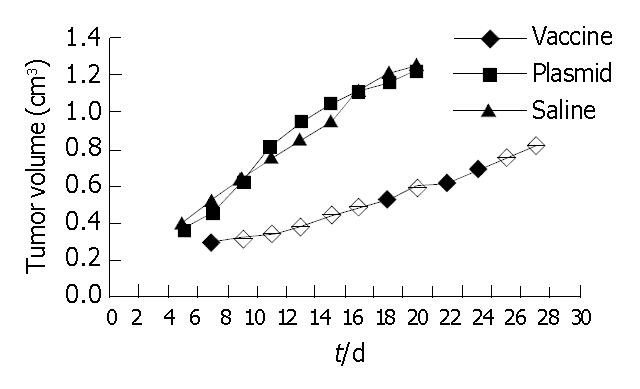
Observation of carcinogenesis Mice of different subgroups in immune preventive group were injected with H22 cells after 3 times inoculation, the tumor latent time, survival time of the vaccine subgroup were longer than that of plasmid subgroup and saline subgroup (P < 0.05); The tumor weight and tumor volume of vaccine subgroup was the lowest among the 3 subgroups (P < 0.05, Table 1, Figure 5). The tumor latent time, survival time, tumor volume and tumor weight of plasmid subgroup and saline subgroup have no statistical significance compared with each other.
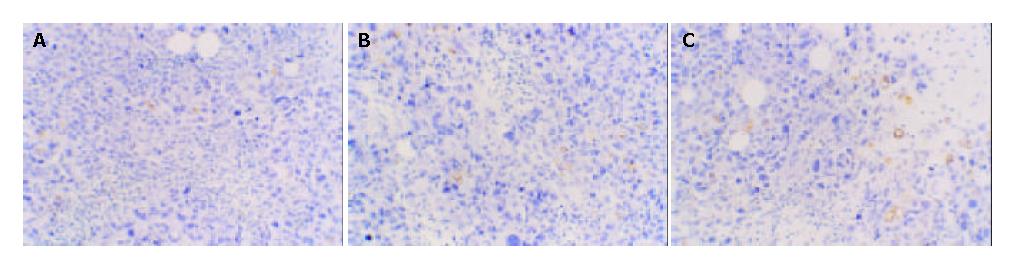
MVD of different subgroups Macroscopic structure showed the tumor tissue was hard and adhered to the surrounding tissue. MVD of vaccine subgroup was the lowest among the three subgroups (P < 0.05). MVD of the plasmid subgroup and saline subgroup have no statistical significance compared with each other (P > 0.05). This indicates that V subgroup has the lowest micro vessel density (Figure 6).
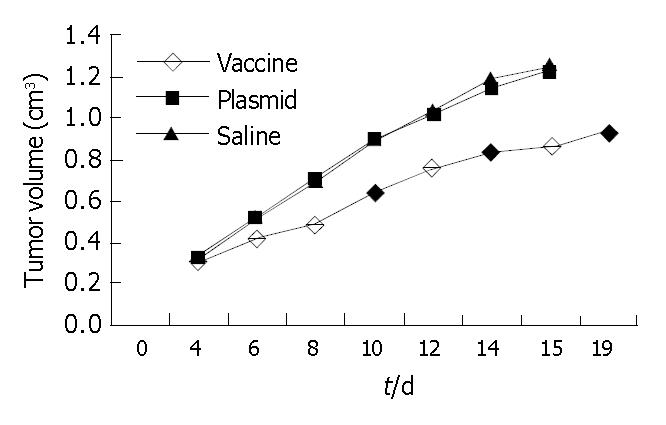
Inhibitory effect of pcDNA3.1 (+)-flk-1-domain1-3 in carcinogenesis Treated with DNA vaccine pcDNA3.1 (+)-flk-1-domain1-3, plasmid and saline, The survival time of the vaccine subgroup were longer than that of plasmid subgroup and saline subgroup (P < 0.05); The tumor weight and tumor volume of vaccine subgroup was the lowest among the 3 subgroups (P < 0.05, Table 2, Figure 7). The survival time, tumor volume and tumor weight of plasmid subgroup and saline subgroup have no statistical significance compared with each other. But the survival times of therapeutic vaccine subgroup were significantly shorter than that of preventive vaccine subgroup (P < 0.05); the tumor size, and MVD of therapeutic vaccine subgroup were significantly greater than that of preventive vaccine subgroup (Table 3).
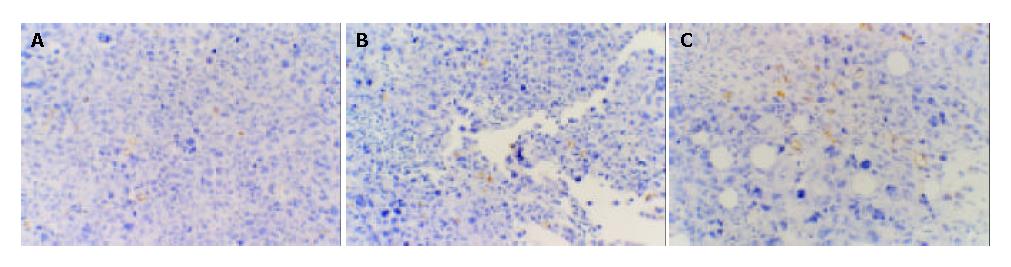
MVD of different subgroups Macroscopic structure showed the tumor tissue was hard and adhered to the surrounding tissue. MVD of vaccine subgroup was the lowest among the 3 subgroups (P < 0.05). MVD of the plasmid subgroup and saline subgroup had no statistical significance compared with each other (Figure 8).
We successfully cloned the domains 1-3 of extracellular parts of flk-1, constructed the DNA vaccine pcDNA3.1 (+) flk-1-domains 1-3, which induced the immune response against endothelial cell, and finally blocked the growth of H22 tumor cells by blocking angiogenesis.
DNA sequencing and blast showed that the cloned gene completely matched the DNA sequence listed in GenBank. Western blot of lipotransfected COS7 showed that the cell transfected with vaccine could express a Mr 44000 specific protein, which had no corresponding band in the control group, indicating that flk-1 could be expressed by the vaccine in eukaryotic cells. Standard 4-h 51Cr release test showed that the vaccine against domains 1-3 could generate specific immune response in mice through breaking tolerance to self-flk-1 antigen.
In the immune preventive group, mice were first inoculated with the vaccine and then challenged with tumor cells. The results showed that compared with the control group, the inoculated mice had a longer survival time, longer tumor latent period, lower tumor weight at death time and slower tumor growth rate. Furthermore, tumor in inoculated mice had low MVD. So we concluded that the vaccine inhibited tumor growth by its anti-angiogenic effects. In the therapeutic group, effects of the vaccine were supposed to investigate in the clinical conditions. The H22 cells were first injected to the mice and waited until tumor was well constructed, then the mice were treated with the vaccine. We found that, similar to the preventive group, the survival time, tumor weight at death time of mice and tumor growth rate all had significant difference compared with the control group. But compared with that of the preventive group, the vaccine was less effective in the therapeutic group, The MVD, survival time had significant difference between the vaccine subgroup of these groups, indicating that the vaccine might be less effective against well-structured tumors.
Recent advances in the understanding of the molecular control of angiogenesis have shown that this process is essential for tumor development and spread. VEGF is a highly specific mitogen for vascular endothelial cells, which can induce endothelial cell proliferation, promotes cell migration, and inhibits cell apoptosis and immune response against cancer[8-11], and plays a central role in the tumor angiogenesis[12-14].
Flk-1 is the main receptor though which VEGF could modulate tumor growth and metastasis[15-18]. Flk-1 belongs to receptor tyrosine kinases (RTKs) family and are expressed almost exclusively in endothelial cells and on various types of tumor cells[19,20]. Flk-1 has a typical tyrosine kinase receptor structure with 7 immunoglobulin (Ig)-like domains in the extracellular region, as well as a long kinase insert in the tyrosine kinase domain[20]. Evidence shows that IgG-like domains 2-3 are sufficient for tight binding, domain 1 is necessary for tight binding and domains 4-7 are not essential for signaling, and the residues within this region of domain 3 are critical for VEGF binding[21,22]. Various studies have shown that VEGF and flk-1 expression are significantly associated with advanced stage, high incidence of distant metastases after surgery, and less favorable prognosis in a number of malignancies[23-28]. As it directly involved in tumor angiogenesis, flk-1 is an appropriate target for suppression of solid tumor growth[6].
Several approaches have been used to block flk-1, including dominant-negative receptor mutants, germ-line disruption of VEGFR genes, monoclonal antibodies, dendritic cell vaccine, and a series of synthetic RTK inhibitors[29-33]. They all achieved the goal of blocking VEGF-flk-1 signaling pathway and inhibiting angiogenesis, but they all have some common defects, such as requiring constant drug injection, only blocking VEGF pathway and high cost. Our strategy was to construct DNA vaccine target flk-1 in order to inhibit angiogenesis by blocking VEGF-flk-1 pathway and destructing endothelial cell that expresses flk-1. Compared with strategies mentioned above, DNA vaccines against flk-1 could achieve similar results and are simpler and cheaper to produce and can induce very long-lasting immune responses[34,35]. Furthermore, the immune response not only blocks VEGF-flk-1 pathway, but also blocks other angiogenesis pathway by destruction of endothelial cells[30]. Also it has been reported that liver cancer cells and various types of other tumor cells could express flk-1 receptor, but in our study we could not amplify flk-1 gene from cultured H22 tumor cells, so we could not evaluate whether or not the vaccine had direct effect on the tumor cells. Our research showed that the vaccine was less effective against well-formed tumors. This may be contributed to the immune escaping capacity of well-structured tumors. This result was in consistent with previous reports at home and abroad[30,35-37]. So we propose that the vaccine should be combined with other therapies, such as surgery, to prevent primary site from recurring and metastasis. Further research is needed in this direction.
In short, we constructed DNA vaccine against extracellular domain1-3 of flk-1, and evaluated its immune preventive and immune therapeutic effects against H22 tumor cell, suggesting that the vaccine could inhibit tumor growth through its anti-angiogenic effect. Further research is needed to increase the effectiveness of this vaccine.
Edited by Kumar M and Chen WW Proofread by Xu FM
| 1. | Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 2002;23:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Shibuya M. [Angiogenesis, anti-angiogenesis, and tumor suppression]. Nihon Yakurigaku Zasshi. 2002;120:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Matter A. Tumor angiogenesis as a therapeutic target. Drug Discov Today. 2001;6:1005-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Bisacchi D, Benelli R, Vanzetto C, Ferrari N, Tosetti F, Albini A. Anti-angiogenesis and angioprevention: mechanisms, problems and perspectives. Cancer Detect Prev. 2003;27:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 352] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Shibuya M. Vascular endothelial growth factor receptor-2: its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003;94:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1299] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 8. | Graff BA, Bjørnaes I, Rofstad EK. Microvascular permeability of human melanoma xenografts to macromolecules: relationships to tumor volumetric growth rate, tumor angiogenesis, and VEGF expression. Microvasc Res. 2001;61:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19:4621-4631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878-4886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 428] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Yoshida S, Amano H, Hayashi I, Kitasato H, Kamata M, Inukai M, Yoshimura H, Majima M. COX-2/VEGF-dependent facilitation of tumor-associated angiogenesis and tumor growth in vivo. Lab Invest. 2003;83:1385-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Verheul HM, Pinedo HM. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin Breast Cancer. 2000;1 Suppl 1:S80-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Werther K, Nielsen HJ. [Significance of vascular endothelial growth factor--VEGF--in tumor angiogenesis. Therapeutic possibilities in solid tumors]. Ugeskr Laeger. 2000;162:4916-4920. [PubMed] |
| 15. | Yoshiji H, Kuriyama S, Hicklin DJ, Huber J, Yoshii J, Miyamoto Y, Kawata M, Ikenaka Y, Nakatani T, Tsujinoue H. KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells. Hepatology. 1999;30:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117-5124. [PubMed] |
| 17. | Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22. [PubMed] |
| 18. | Xiang F, Tanaka J, Takahashi J, Fukuda T. Expression of vascular endothelial growth factor (VEGF) and its two receptors in diffusely infiltrating astrocytomas and relationship to proliferative activity of tumor cells. Brain Tumor Pathol. 2001;18:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5 Suppl 1:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem. 1998;273:31283-31288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Lu D, Kussie P, Pytowski B, Persaud K, Bohlen P, Witte L, Zhu Z. Identification of the residues in the extracellular region of KDR important for interaction with vascular endothelial growth factor and neutralizing anti-KDR antibodies. J Biol Chem. 2000;275:14321-14330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Rydén L, Linderholm B, Nielsen NH, Emdin S, Jönsson PE, Landberg G. Tumor specific VEGF-A and VEGFR2/KDR protein are co-expressed in breast cancer. Breast Cancer Res Treat. 2003;82:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Mukherjee T, Kumar A, Mathur M, Chattopadhyay TK, Ralhan R. Ets-1 and VEGF expression correlates with tumor angiogenesis, lymph node metastasis, and patient survival in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2003;129:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Stockhammer G, Obwegeser A, Kostron H, Schumacher P, Muigg A, Felber S, Maier H, Slavc I, Gunsilius E, Gastl G. Vascular endothelial growth factor (VEGF) is elevated in brain tumor cysts and correlates with tumor progression. Acta Neuropathol. 2000;100:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Wang S, Xia T, Zhang Z, Kong X, Zeng L, Mi P, Xue Z. [Expression of VEGF and tumor angiogenesis in bladder cancer]. Zhonghua Waike Zazhi. 2000;38:34-36. [PubMed] |
| 27. | Tamura M, Ohta Y, Kajita T, Kimura K, Go T, Oda M, Nakamura H, Watanabe G. Plasma VEGF concentration can predict the tumor angiogenic capacity in non-small cell lung cancer. Oncol Rep. 2001;8:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Karademir S, Sökmen S, Terzi C, Sağol O, Ozer E, Astarcioğlu H, Coker A, Astarcioğlu I. Tumor angiogenesis as a prognostic predictor in pancreatic cancer. J Hepatobiliary Pancreat Surg. 2000;7:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Becker CM, Farnebo FA, Iordanescu I, Behonick DJ, Shih MC, Dunning P, Christofferson R, Mulligan RC, Taylor GA, Kuo CJ. Gene therapy of prostate cancer with the soluble vascular endothelial growth factor receptor Flk1. Cancer Biol Ther. 2002;1:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Li Y, Wang MN, Li H, King KD, Bassi R, Sun H, Santiago A, Hooper AT, Bohlen P, Hicklin DJ. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med. 2002;195:1575-1584. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002;5:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Shibuya M. VEGF-receptor inhibitors for anti-angiogenesis. Nihon Yakurigaku Zasshi. 2003;122:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Haupt K, Roggendorf M, Mann K. The potential of DNA vaccination against tumor-associated antigens for antitumor therapy. Exp Biol Med (Maywood). 2002;227:227-237. [PubMed] |
| 34. | Henke A. DNA immunization--a new chance in vaccine research. Med Microbiol Immunol. 2002;191:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Muehlbauer PM. Anti-angiogenesis in cancer therapy. Semin Oncol Nurs. 2003;19:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Zogakis TG, Libutti SK. General aspects of anti-angiogenesis and cancer therapy. Expert Opin Biol Ther. 2001;1:253-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Takahashi Y, Mai M. [Significance of angiogenesis and clinical application of anti-angiogenesis]. Nihon Geka Gakkai Zasshi. 2001;102:381-384. [PubMed] |