Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2019
Revised: September 18, 2003
Accepted: October 12, 2003
Published online: July 15, 2004
AIM: To study the effects of sense and antisense KAI1 genes on the growth and invasion of human hepatocellular carcinoma (HCC) cell line MHCC97-H.
METHODS: KAI1 sense and antisense eukaryotic expression plasmids were constructed using subclone technique and transfected into MHCC97-H cells respectively by DOTAP liposome. After successful transfection was confirmed, in vitro growth curve, cell cycles, plate clone formation efficiency, invasive ability in Boyden Chamber assay and ultrastructural morphology were studied.
RESULTS: KAI1 sense and antisense genes had no significant effects on the cell growth curve and cell cycles. After transfection with sense KAI1 gene, decreased invasive ability in Boyden Chamber assay and decreased amount of mitochondria, but no significant changes of plate clone formation efficiency were observed in MHCC97-H-S cells. The plate clone formation efficiency and invasive ability in Boyden Chamber assay were significantly increased in MHCC97-H-AS cells, after transfection with antisense KAI1 gene. Furthermore, increased amount of mitochondria, rough endoplasmic reticulum, Golgi apparatus and expanded endoplasmic reticulum were also noted in MHCC97-H-AS cells.
CONCLUSION: Changes of KAI1 expression in HCC cells may alter their invasive and metastasis ability of the tumor.
- Citation: Si SH, Yang JM, Peng ZH, Luo YH, Zhou P. Effects of KAI1 gene on growth and invasion of human hepatocellular carcinoma MHCC97-H cells. World J Gastroenterol 2004; 10(14): 2019-2023
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2019.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2019
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China. Metastasis and recurrence are the most principal factors for the prognosis of patients with the tumor[1]. KAI1 gene, isolated from human metastatic prostate tumor in 1995, was regarded as a new metastasis suppressor gene. Up to now, many researches on the relationship between KAI1 gene and invasion, metastasis of malignant tumors have been reported, but most of them were made by histopathological and molecular pathological methods[2-16]. In the present research, we studied the effects of KAI1 gene on the growth and invasion of human hepatocellular carcinoma (HCC) by subclone, gene transfection and antisense technology.
Plasmid pCMV-KAI1, with a full length of 8.2 kb, containing human a full-length of KAI1 structural cDNA gene, was a generous gift from Professor J.Carl Barrett and Dr. Dong of National Institute of Health of USA[2]. Eukaryotic expression plasmid vector pCI-neo, with a full length of 4.7 kb, was purchased from Promega Corporation.
Sense and antisense KAI1 eukaryotic expression plasmids constructed by subclone technique, were confirmed and identified by restriction endonuclease Sal I and Xba I analysis.
MHCC97-H, a hepatocellular carcinoma cell line with highly metastatic potential[17] , purchased from Liver Cancer Institute, Zhongshan Hospital,Shanghai Fudan University, was used as target cells of gene transfection in the present study. The cell line was cultured in Dulbecco minimum essential medium (Hyclone, USA) containing 100 mL/L fetal calf serum (Hyclone, USA), 100 kU/L penicillin and 100 kU/L streptomycin at 37 °C in a 50 mL/L CO2 incubator.
Sense and antisense KAI1 eukaryotic expression plasmids were transfected into MHCC97-H cells respectively by DOTAP (Roche, USA) liposome transfection system similar to our previous report[18]. Forty-eight hours after transfection, the cells were transfer-selection-cultured for two weeks under the pressure of 800 mg/L G418 (Pierces, USA) in the culture medium. Then the positive cell clones were mixed and the resistance cells were expanded to be cultured under the pressure of 250-600 mg/L G418. Enhanced culture, passaging and storage were performed until few cells were killed by G418.
Gene integration was identified with amplification of neo genes[19] by polymerase chain reaction (PCR). The primers, designed to amplify the neo genes in vector pCI-neo: forward (P1): 5’-CAA GAT GGA TTG CAC GCA GG-3’, reverse (P2): 5’-CCC GCT CAG AAG AAC TCG TC -3’, were synthesized by Shanghai Bioenginering Company, China. Theoretical length of PCR product was 790 bp. Western blot and immunocytochemistry were also performed to understand whether transfection was successful and the effects of transfected genes on KAI1 expression in MHCC97-H cells.
Ultrastructural changes of the cells transfected with sense or antisense KAI1 gene were observed with transmission electron microscope.
Cells in exponential growth phase were trypsinized to develop single-cell suspension. Four × 104 viable cells in 1 mL of medium were added to each well of the 24-well culture plates, which were incubated at 37 °C with 50 mL/L CO2. Cell numbers in 4 wells were counted and averaged with blood counting chamber every 24 h for 10 consecutive days, and cell growth curve was plotted based on these results.
Cell cycle analyses were performed by flow cytometry, and each group was examined for 3 times.
Cells cultured (2 × 103) for 48 h were added to each well containing 1mL of culture medium of a 6-well culture plate, each cell group contained 4 wells. Then the culture plate was gently swayed to disperse cells. The cells were incubated at 37 °C with 50 mL/L CO2 for 12 d. Then the cells were washed twice with warm PBS, and stained with Giemsa solution. The number of colonies containing 50 cells or more was counted under a microscope.
In vitro invasive ability was tested by Boyden Chamber assay[20,21] according to the kit directions. Invasion Chamber inserts (BD Biosciences, USA) with 8 μm-pores in their PET membrane had been coated by matrigel. Before invasion assay, the invasion chambers were rehydrated with DMEM (serum-free) for 2 h in a humidified tissue culture incubator at 37 °C with 50 mL/L CO2 atmosphere. DMEM with 100 mL/L fetal bovine serum was added to the lower compartment, and 1.5 × 105 tumor cells in serum-free DMEM were added to the upper compartment of the chamber. Each cell group was plated in 3 duplicate wells. After 48 h incubation, the matrigel was removed, and the filter was washed, cells were fixed and stained in Giemsa solution. Then the cells having migrated to the lower sides of the PET membrane in 5 random visual fields (200 ×) were counted under a light microscope.
Statistical analysis software package SPSS (V10.0) was used for data processing, and P < 0.05 was considered statistically significant.
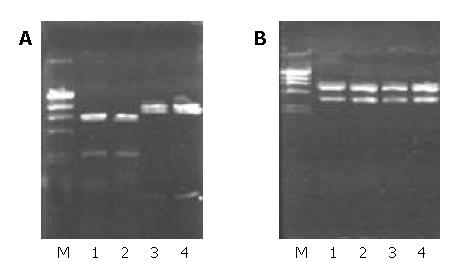
Two fragments of 3 kb and 5.2 kb appeared after pCMV-KAI1 was digested by Xba I. According to the plasmid pCMV-KAI1 map, the 3 kb fragment containing human full-length structural KAI1 cDNA gene was extracted. The cleaved vector pCI-neo after digestion by Xba I and dephosphorylation by CIP was linked up with the 3 kb fragment. Two recombinant plasmids were extracted and digested by Sal I, the plasmid appearing 2 fragments of 2.25 kb and 6.22 kb after digestion was consistent with the expected sense KAI1 expression plasmid, and the other appearing two fragments of 0.75 kb and 7.72 kb was consistent with the expected antisense KAI1 expression plasmid (Figure 1A). Then the 2 recombinants were digested by Xba I to further confirm the insertion of target fragment into the vector. The results showed the two recombinants were respectively cleaved into the same two fragments 3kb, 5.4 kb (Figure 1B), and suggested subclone reconstruction was successfully performed.
The recombinants containing sense or antisense KAI1 cDNA were respectively transfected into MHCC97-H cells by DOTAP liposome system. After stable cell clones were screened by G418, the cells transfected with sense KAI1 gene were renamed as MHCC97-H-S, the cells transfected with antisense KAI1 gene were renamed as MHCC97-H-AS, and the cells transfected with vector pCI-neo (vacant vector) were renamed as MHCC97-H-pCI. With amplification of the neo genes by PCR, a specific fragment with a length of 790 bp could be produced from MHCC97-H-S, MHCC97-H-AS and MHCC97-H-pCI cells, but none from MHCC97-H cells (Figure 2). It suggested that the recombinant genes were integrated into genomes of the target cells respectively.
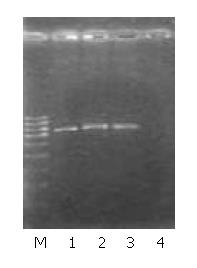
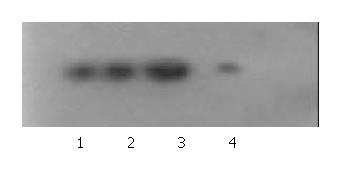
Western blot and immunocytochemistry staining showed KAI1 protein expression was enhanced in MHCC97-H-S, but decreased in MHCC97-H-AS, and no obvious difference in MHCC97-H-pCI as compared with MHCC97-H cells (Figure 3, Figure 4).
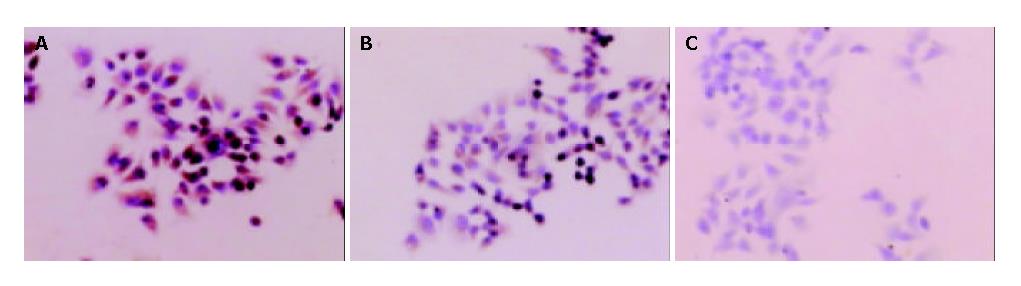
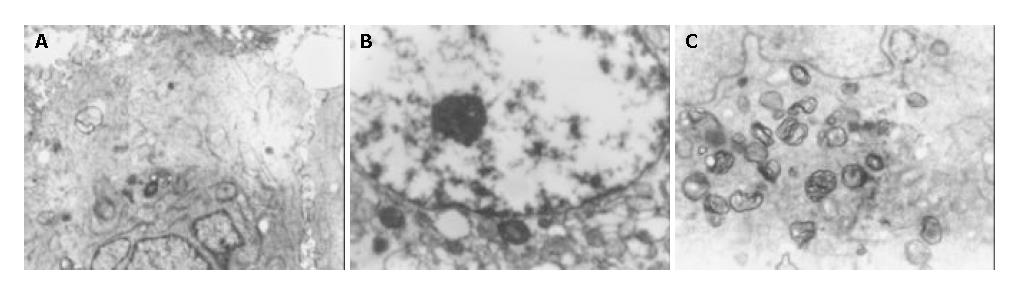
Under electron microscope, the amount of mitochondria was decreased in MHCC97-H-S cells, but increased in MHCC97-H-AS cells. Furthermore, increased amount of rough endoplasmic reticulum (RER), Golgi apparatus, expanded endoplasmic reticulum and myelin-sheath-like changes of mitochondria were also noted in MHCC97-H-AS cells (Figure 5). However, there were no obvious differences in cell shape, superficial microvilli, karyotype and karyokinesis among MHCC97-H-S, MHCC97-H-AS and MHCC97-H cells.
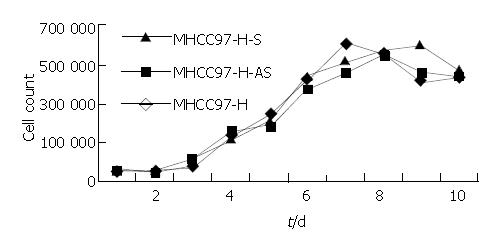
No significant differences in growth curve and cell cycles were observed among MHCC97-H-S, MHCC97-H-AS, and MHCC97-H cells in 10 consecutive days (Figure 6, Table 1). The results demonstrated that KAI1 gene had no obvious effect on cell proliferation ability.
| Cell | G0/G1 | S | G2/M |
| MHCC97-H-S | 69.8 ± 6.7 | 19.7 ± 6.4 | 10.5 ± 0.9 |
| MHCC97-H-AS | 72.1 ± 7.2 | 19.0 ± 6.3 | 8.9 ± 0.9 |
| MHCC97-H | 73.1 ± 2.0 | 16.4 ± 1.4 | 10.5 ± 1.5 |
After two weeks of culture, the results of plate clone formation test showed no statistical difference in clone formation number between MHCC97-H-S (36.2 ± 3.3) and MHCC97-H (38.5 ± 1.9) (P > 0.05). However, MHCC97-H-AS (132.5 ± 2.9) showed more clone formation number (P < 0.01).
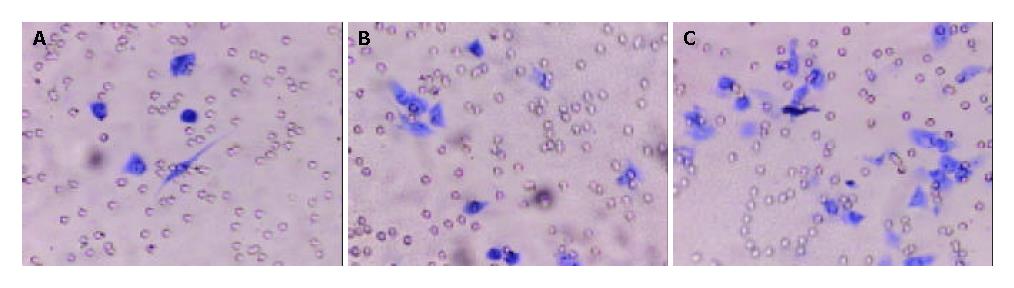
Boyden Chamber assay showed that the cells penetrated the artificial basement membrane in MHCC97-H-S (59.7 ± 3.5) were fewer than in MHCC97-H (92.7 ± 1.5) (P < 0.01). However, more penetrated cells in MHCC97-H-AS (188.00 ± 4.5) were noted as compared with those in MHCC97-H (P < 0.01). These results suggested that sense KAI1 gene could decrease the invasive ability of MHCC97-H, while, antisense KAI1 gene could increase the invasive ability of MHCC97-H (Figure 7).
KAI1 is a newly discovered metastasis suppressor gene in human prostate cancer, which was mapped to chromosome 11p11.2 by Dr. Dong et al[2] of National Institute of Health, USA in 1995. KAI1 specifies a leukocyte surface glycoprotein of 267 amino acids with molecular mass of 29610 u. KAI1 protein has been recognized as a structurally distinct family of membrane glycoproteins, transmembrane 4 superfamily[22]. Recent researches showed the expression of KAI1 was down-regulated not only in human prostate cancer but also in a variety of human malignant tumors[3,16], and these suggested KAI1 might be a broad-spectrum metastasis suppressor gene for human tumors.
Previous studies on the relationship between KAI1 gene and tumor invasion, metastasis were mostly based on the detection of KAI1 protein, DNA or RNA in tissue samples from surgical resections with immunopathological and molecular pathological methods in combination with analysis of clinical data. In the present study, we transfected full-length structural sense and antisense KAI1 genes from subclone recombinants into hepatocellular carcinoma MHCC97-H cell line with highly metastatic potential respectively. By observation of the changes of cell growth, invasion and ultrastructures of MHCC97-H before and after KAI1 transfection, we hoped to understand the effects of KAI1 gene on the capability of growth and invasion of hepatocellular carcinoma and to provide experimental evidences for the prevention and treatment of invasion and metastasis of this malignant tumor.
Restriction endonuclease analysis showed KAI1 full-length cDNA was respectively inserted into plasmid vector pCI-neo by two different directions, indicating that sense and antisense KAI1 genes eukaryon expression plasmids were successfully reconstructed. After KAI1 gene transfected into MHCC97-H by DOTAP liposome system, amplification of the neo gene in transfected cells was performed by PCR to confirm whether KAI1 gene was integrated into genomes of target MHCC97-H cells. Neo is a screening marker gene in the vector pCI-neo, and no sequence of this gene exists in the genomes of mammal cells, but it could stably exist in the genomes of mammal cells after transfection. Therefore, if the sequence could be amplified from the genomes of transfected cells by PCR, it could confirm the exogenous genes were integrated into genomes of MHCC97-H cells. PCR results showed sense and antisense KAI1 genes eukaryon expression plasmids were successfully integrated into genomes of MHCC97-H. Western blot and immunocytochemical staining were made to determine if the recombinant plasmids could expectantly express in the transfected cells. The results showed that sense KAI1 could up regulate the expression of KAI1 protein in MHCC97-H as compared with the control parental cells, but antisense KAI1 could down regulate the expression of KAI1 protein, and no obvious difference between the cells transfected with pCI-neo and the control parental cells was noted. These results showed the transfected genes could produce expected effects, and vector pCI-neo itself had no obvious effects on the expression of KAI1.These suggested that the above cells transfected with genes could be used to explore the effects of KAI1 on the biological behaviors of HCC cells.
In the present study, our data indicated that KAI1 gene had no obvious effects on the in vitro cell growth and proliferation of hepatocellular carcinoma MHCC97-H cell line. These results were consistent with the studies in prostatic cancer, colon cancer cells by Dong and Takaoka et al[2,23]. In contrast with MHCC97-H control parent cells, the plate clone formation ability had no obvious reduction in MHCC97-H-S, but had obvious enhancement in MHCC97-H-AS cells. These data suggested down regulated expression of KAI1 could enhance motoricity and aggregative abilities of MHCC97-H. Though the cells transfected with sense KAI1 expressed more KAI1 protein revealed by Western blot and immunocytochemical staining, their plate clone formation ability had no obvious reduction. This may be attributed to the small amount of KAI1 protein expression in MHCC97-H cells.
Invasion has been found to be one of the important and necessary properties for tumor metastasis formation[24-29]. Boyden chamber assay, which imitates the invasion process of in vivo tumor cells, has also been found to be an ideal method for evaluating the invasive and metastatic abilities of tumor cells[20,22]. Our data suggested that the invasive ability of HCC cells transfected with antisense KAI1 gene was increased, but the invasive ability of HCC cells transfected with sense KAI1 gene was partly inhibited. These indicated that different levels of KAI1 protein expression in HCC cells could affect their invasive and metastatic ability and that it might be an effective route to up regulate KAI1 expression for inhibiting the metastasis of HCC.
Under transmission electron microscope, we found that though KAI1 gene had no obvious effects on cell shape, superficial microvillus, karyotype and karyokinesis of MHCC97-H, sense KAI1 gene transfected into MHCC97-H resulted in reduction of the amount of mitochondria, however antisense KAI1 gene transfection resulted in increase of the amount of mitochondria, RER and Golgi apparatus with expanded endoplasmic reticulum and myelin-sheath-like changes of mitochondria. The biological function of these changes was unknown. It is still necessary to further study whether increased expression of KAI1 can inhibit invasion and metastasis of tumor cells by decreasing products of some organelles.
Though present researches have suggested KAI1 gene is important in preventing the development of metastases in a wide variety of human tumors, the mechanism of KAI1-mediated metastasis suppression remains unclear. The molecular structure of KAI1 protein indicates KAI1 functions in cell-cell interactions and cell-extracellular matrix interactions. These suggest that KAI1 may affect cell-cell adhesion and cell-extracellular matrix adhesion through some signaling pathways to inhibit tumor metastasis[30,31].
Co-correspondents: Dr. Sui-Hai Si
Edited by Xu CT and Wang XL Proofread by Pan BR and Xu FM
| 1. | Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193-199. [PubMed] |
| 2. | Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 546] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998;153:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | White A, Lamb PW, Barrett JC. Frequent downregulation of the KAI1(CD82) metastasis suppressor protein in human cancer cell lines. Oncogene. 1998;16:3143-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Adachi M, Taki T, Konishi T, Huang CI, Higashiyama M, Miyake M. Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J Clin Oncol. 1998;16:1397-1406. [PubMed] |
| 6. | Yu Y, Yang JL, Markovic B, Jackson P, Yardley G, Barrett J, Russell PJ. Loss of KAI1 messenger RNA expression in both high-grade and invasive human bladder cancers. Clin Cancer Res. 1997;3:1045-1049. [PubMed] |
| 7. | Houle CD, Ding XY, Foley JF, Afshari CA, Barrett JC, Davis BJ. Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol Oncol. 2002;86:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Liu FS, Chen JT, Dong JT, Hsieh YT, Lin AJ, Ho ES, Hung MJ, Lu CH. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol. 2001;159:1629-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Liu FS, Dong JT, Chen JT, Hsieh YT, Ho ES, Hung MJ, Lu CH, Chiou LC. KAI1 metastasis suppressor protein is down-regulated during the progression of human endometrial cancer. Clin Cancer Res. 2003;9:1393-1398. [PubMed] |
| 10. | Wu DH, Liu L, Chen LH, Ding YQ. Expression of KAI1/CD82 in human colorectal tumor. Diyi Junyi Daxue Xuebao. 2003;23:714-715, 719. [PubMed] |
| 11. | Ito Y, Yoshida H, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Kuma K. KAI1 expression in thyroid neoplasms: its linkage with clinicopathologic features in papillary carcinoma. Pathol Res Pract. 2003;199:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Yang JL, Jackson P, Yu Y, Russell PJ, Markovic B, Crowe PJ. Expression of the KAI1 metastasis suppressor gene in non-metastatic versus metastatic human colorectal cancer. Anticancer Res. 2000;22:3337-3342. [PubMed] |
| 14. | Tozawa K, Akita H, Kawai N, Okamura T, Sasaki S, Hayashi Y, Kohri K. KAI1 expression can be a predictor of stage A prostate cancer progression. Prostate Cancer Prostatic Dis. 2001;4:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Imai Y, Sasaki T, Shinagawa Y, Akimoto K, Fujibayashi T. Expression of metastasis suppressor gene (KAI1/CD82) in oral squamous cell carcinoma and its clinico-pathological significance. Oral Oncol. 2002;38:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Muneyuki T, Watanabe M, Yamanaka M, Shiraishi T, Isaji S. KAI1/CD82 expression as a prognosic factor in sporadic colorectal cancer. Anticancer Res. 2001;21:3581-3587. [PubMed] |
| 17. | Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630-636. [PubMed] |
| 18. | Yang JM, Chen WS, Liu ZP, Luo YH, Liu WW. Effects of insulin-like growth factors-IR and -IIR antisense gene transfection on the biological behaviors of SMMC-7721 human hepatoma cells. J Gastroenterol Hepatol. 2003;18:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Poggiali P, Scoarughi GL, Lavitrano M, Donini P, Cimmino C. Construction of a swine artificial chromosome: a novel vector for transgenesis in the pig. Biochimie. 2002;84:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Nawrocki-Raby B, Gilles C, Polette M, Bruyneel E, Laronze JY, Bonnet N, Foidart JM, Mareel M, Birembaut P. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer. 2003;105:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Vehviläinen P, Hyytiäinen M, Keski-Oja J. Latent transforming growth factor-beta-binding protein 2 is an adhesion protein for melanoma cells. J Biol Chem. 2003;278:24705-24713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Dong JT, Isaacs WB, Barrett JC, Isaacs JT. Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics. 1997;41:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, Imai K. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene. 1998;16:1443-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Kurokawa H, Katsube K, Podyma KA, Ikuta M, Iseki H, Nakajima M, Akashi T, Omura K, Takagi M, Yanagishita M. Heparanase and tumor invasion patterns in human oral squamous cell carcinoma xenografts. Cancer Sci. 2003;94:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Wernicke M, Piñeiro LC, Caramutti D, Dorn VG, Raffo MM, Guixa HG, Telenta M, Morandi AA. Breast cancer stromal myxoid changes are associated with tumor invasion and metastasis: a central role for hyaluronan. Mod Pathol. 2003;16:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Katayama M, Sanzen N, Funakoshi A, Sekiguchi K. Laminin gamma2-chain fragment in the circulation: a prognostic indicator of epithelial tumor invasion. Cancer Res. 2003;63:222-229. [PubMed] |
| 27. | Wells A, Kassis J, Solava J, Turner T, Lauffenburger DA. Growth factor-induced cell motility in tumor invasion. Acta Oncol. 2002;41:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Jee B, Jin K, Hahn JH, Song HG, Lee H. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp Mol Med. 2003;35:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Shibagaki N, Hanada Ki, Yamashita H, Shimada S, Hamada H. Overexpression of CD82 on human T cells enhances LFA-1 / ICAM-1-mediated cell-cell adhesion: functional association between CD82 and LFA-1 in T cell activation. Eur J Immunol. 1999;29:4081-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |