Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2010
Revised: February 11, 2004
Accepted: February 21, 2004
Published online: July 15, 2004
AIM: To evaluate the effect of aclacinomycin A-loaded magnetic polybutylcyanoacrylate nanoparticles on gastric tumor growth in vivo and in vitro.
METHODS: Magnetic polybutylcyanoacrylate (PBCA) nanospheres encapsulated with aclacinomycin A (MPNS-ACM) were prepared by interfacial polymerization. Particle size, shape and drug content were examined. Female BABL/c nude mice were implanted with MKN-45 gastric carcinoma tissues subcutaneously to establish human gastric carcinoma model. The mice were randomly divided into 5 groups of 6 each: ACM group (8 mg/kg bm); group of high dosage of MPNS-ACM (8 mg/kg bm); group of low dosage of MPNS-ACM (1.6 mg/kg bm); group of magnetic PBCA nanosphere (MPNS) and control group (normal saline). Magnets (2.5 T) were implanted into the tumor masses in all of the mice one day before the therapy. Above-mentioned drugs were administered intravenously to the mice of every group on the first day and sixth day. When the mice were sacrificed, tumor weight was measured, and the assay of granulocyte- macrophage colony forming-unit (CFU-GM) was performed on semi-solid culture. White blood cell, alanine aminotransferase and creatine were examined. 3-[4-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was used to examine the viability of MKN-45 cells after incubation with different concentrations of ACM, MPNS and MPNS-ACM suspension respectively for 48 h.
RESULTS: Content of ACM in MPNS-ACM was 12.0% and the average diameter of the particles was 210 nm. The inhibitory rates of ACM (8 mg/kg bm), high dosage of MPNS-ACM (8 mg/kg bm), low dosage of MPNS-ACM (1.6 mg/kg bm) and MPNS on human gastric carcinoma in nude mice were 22.63%, 52.55%, 30.66% and 10.22%, respectively. There was a significant decrease in the number of CFU-GM of bone marrow in ACM group compared with control group, whereas no obvious change was observed in that of the nanosphere groups. The values of 50% inhibition concentration (IC50) of ACM, MPNS and MPNS-ACM were 0.09, 97.78 and 1.07 μg/mL, respectively.
CONCLUSION: The tumor inhibitory rate of MPNS-ACM was much higher than that of ACM under magnetic field and the inhibition on bone marrow was alleviated significantly compared with ACM group.
- Citation: Gao H, Wang JY, Shen XZ, Deng YH, Zhang W. Preparation of magnetic polybutylcyanoacrylate nanospheres encapsulated with aclacinomycin A and its effect on gastric tumor. World J Gastroenterol 2004; 10(14): 2010-2013
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2010.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2010
Cytotoxic medicine has extensively been employed in cancer chemotherapy. However, the usage of these drugs has been limited by the non-targeting towards cancer and serious toxicity to normal cells in the body. To enhance the therapeutic efficacy of anticancer drugs and reduce the toxicity to normal tissue of the body, targeted drug delivery systems at solid tumors have been developed.
Magnetic targeted drugs are the fourth generation of targeted reagents. The advantage of the magnetic targeted drug delivery systems over other drug targeting techniques is their ability to minimize the uptake by reticuloendothelial system (RES)[1]. Some investigators have reported successful tumor remission in animal experiments upon the use of magnetically responsive anticancer drug carriers under magnetic fields, but in previous studies the majority of magnetically responsive drug carriers, which included magnetic albumin microspheres and magnetic liposomes had been administered intra-arterially to obtain highly efficient localized targeting during the first circulation passing through a strong magnetic field[2,3]. However intra-arterial administration of these carriers is not considered to be suitable for the treatment of multiple systemic lesions and is inconvenient to apply. Accordingly, the treatment of solid tumors requires the development of magnetically responsive carriers that can be effectively delivered to any systemic site via intravenous administration. Yet the therapeutic efficacy of intravenously administered magnetically responsive carriers has not been estalished maturely to date.
Polyalkylcyanoacrylates (PACAs) were not employed as polymers until the early 1980s. However the corresponding monomers, alkylcyanoacrylates, have been extensively used as tissue adhesives for the closure of skin wounds[4]. More recently, one application of the polymers is the use of PACAs as nanoparticulate drug carriers[5-9]. This very exciting area of research has gained increasing interest in therapeutics, especially for cancer treatments. Other molecules of interest, including poorly stable compounds such as peptides and nucleic acids, have been combined with PACAs nanoparticles for targeting purposes[10]. Today, PACAs nanoparticles are considered the most promising polymer colloidal for drug delivery system and are already in clinical development for cancer therapy[11-15]. The main attraction of PACAs nanoparticles is their ability of tissue targeting and enhanceing intracellular penetration of drugs[16]. Among the species, polybutylcyanoacrylate (PBCA), as a polymer with medium-length alkyl side chain, is of lower toxicity, proper degradation time. PBCA carrying drugs could increase the antibacterial efficacy[17], elevate the anti-cancer effect[18], enhance the relative bioavailability of insulin[19] etc. So PBCA has recently been regarded as a kind of widely used, biocompatible, degradable, low-toxic drug carrier.
Employing supermagnetic iron oxide as the ferromagnetic material, aclacinomycin A (ACM) as the targeted fat soluble model drug and PBCA as the carrier, a kind of magnetically targeted polymer encapsulated with an anticancer drug, magnetic PBCA nanospheres encapsulated with ACM were designed and successfully prepared. The antiancer efficacy of the magnetically targeted system was investigated on gastric cancer in vivo and in vitro.
Butylcyanoacrylate was supplied by Zhejiang Golden Roc. Chemicals Co. Inc. ACM was obtained from Shenzhen Main Luck Pharmaceuticals Inc. Human gastric cancer MKN-45 cell line and BALB/c nude mice (SPF, female) were kindly supplied by Shanghai Cancer Institute. Column NdFeB permanent magnets (surface field strength 2.5 T, diameter 1 mm, length 10 mm) were supplied by Shanghai Jieling Magnetic & Device Co. Ltd. Semi-solid methylcellulose M3545 and Iscove’s Modified Dulbecco’s Medium (IMDM) were purchased from Stemcell Company, Canada.
Synthesis of magnetic colloidal nanoparticles Magnetic colloidal iron oxide nanoparticles were prepared with the method of coprecipitation as described before[20]. Briefly, 10 mol/L sodium hydroxide was added into the mixture of solution of FeCl2 and FeCl3 (Fe2+/Fe3+ molar ratio 1/2) in a nitrogen atmosphere. The solution was stirred for 1 h at 20 °C, and heated at 90 °C for 1 h. The obtained iron oxide suspension was then stirred for 30 min at 90 °C with the addition of 100 mL trisodium citrate solution (0.3 mol/L). Subsequently, the iron oxide dispersion was cooled down to room temperature with continuous stir. The magnetic particles were washed with double distilled deionized water and collected with the help of a magnet. Finally, the ultrafine magnetic particles were redispersed in water and the suspensions were adjusted to 2.0% (w/w) for further use.
Preparation of ACM encapsulated magnetic PBCA nanoparticles (MPNS-ACM) Interfacial polymerization was applied to synthesize MPNS-ACM based on the methods used before[21,22]. Briefly, 20 mg ACM dispersed in diluted hydrochloric acid and 2 mL magnetic fluid were mingled, then the mixture was added to 100 g hexane including 2 g Span 80 and 0.6 g polysorbate 80 which was stirred at 600 r/min. The fluid was stirred for 0.5-1 h to make it uniform and emulsive. Then 2 mL butylcyanoacrylate was added dropwise with constant stir at room temperature. After 6 h polymerization the particles were separated with the help of a magnet and washed with methanol for several times. Then the particles were washed with deionized water for several times. The particles were lyophilized and 60Co irradiation (15 kGy) was performed to sterilize them. The preparation of magnetic PBCA nanoparticles (MPNS) is similar to the synthesis of MPNS-ACM except no ACM in HCl solution.
Characterization and measurements Transmission electron microscopy (TEM) was performed for MPNS (Hitachi HU-11B). Scanning electron microscopy (SEM, Philips XL30) was used to determine the size and morphology of MPNS. Dynamic light scattering (DLS, Malvern 4700) was used to measure the hydrodynamic diameter of nanoparticles. The particles were treated with ammonia and then ACM was extracted with ethyl acetate. ACM concentration was determined with UV spectrophotometer at 259 nm. And then drug contents were calculated.
Human gastric carcinoma model of nude mice Human gastric carcinoma MKN-45 cells during exponential growth phase were adjusted to 5 × 107 /mL with RPMI 1640. Then 0.2 mL suspension of MKN-45 cells was inoculated subcutaneously near right forefoot in female BALB/c nude mice. Two weeks later, the solid tumors were taken out from the mice in which the tumors were well growing without necrosis. Tumor masses were cut into small pieces with the diameter of about 2 mm. One tiny piece was implanted into one mouse subcutaneously near right forefoot with a needle. The models were successfully produced after about 2 wk when the tumor grew up to 1 cm in diameter.
Tumor inhibition rate in vivo Thirty nude mice models were randomly divided into 5 groups of six each: ACM group (8 mg/kg bm), high dose of MPNS-ACM group (equivalent to ACM 8 mg/kg bm), low dose of MPNS-ACM group (equivalent to ACM 1.6 mg/kg bm), MPNS carrier group (equivalent to MPNS-ACM loaded with ACM 8 mg/kg bm) and control group (normal saline). Magnets (with surfacial field strength 2.5 T) were implanted into the center of the tumors one day before the administration. Above-mentioned agents were administrated intravenously on the first day and sixth day. The largest diameter (LD) and its vertical diameter (VD) of the tumors were measured with calipers every two days after the beginning of administration. The volume of tumor was equal to LD × VD2/2. The mice were sacrificed on the eleventh day after treatment. The tumors were taken out, weighed and the tumor inhibition rate (TIR) was calculated with the following formula: TIR(%) = (1 - average tumor weight of experimental group/average tumor weight of control group) × 100%.
Stem cells colony-forming unit assay of bone marrow was performed. White blood cell, serum alanine aminotransferase (ALT) and creatine of the mice were examined.
Assay of granulocyte-macrophage colony-forming unit (CFU-GM) of bone marrow The assay of CFU-GM was performed with semi-solid methylcellulose culture. The femoras of the mice were taken out under sterile condition. Both extremities of them were cut and the bones were immersed into Iscove’s modified Dulbecco’s medium (IMDM). The bone marrow was washed out and the concentration of the cells with nuclei was adjusted with IMDM to 2 × 105/mL. Cell suspension 0.2 mL was added to 2 mL M3534 semi-solid culture. The mixture was added to a 12-well cell culture plate. The cells were cultured for 7 d at 37 °C with 50 mL/L CO2 in air and > 95% humidity. The number of colonies (> 30 cells) were counted under inverted microscope[23].
Anticarcinoma effect on gastric cancer cell line in vitro
Gastric cancer cell line MKN-45 cells were trypsinized and suspended in RPMI 1640 with the concentration of 2 × 105/mL. The cells were seeded onto 96-well culture plates with 190 µL per well and then were cultured at 37 °C with 50 ml/L CO2 in air and > 95% humidity for 24 h. Different concentrations of ACM, MPNS and MPNS-ACM in RPMI 1640 were added to the wells and the final concentrations were 0.001, 0.01, 0.1, 1.0, 10 and 100 µg/mL, respectively. The cells were cultured for another 48 h. Then, 10 µL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylterazolium bromide (MTT) was added to each well and the cells were cultured for 4 h at 37 °C. Then the culture medium was discarded, and 150 µL dimethylsulfoxide was added to each well and the absorbance at 570 nm (A570) was measured with microplate reader.
Inhibition rate = (1 - A570 in treatment group/A570 in control group) × 100%.
The data were presented as mean ± SD. One-Way ANOVA analysis was used to perform single factor multiple comparison in animal tests. The level of significance was set at P < 0.05. Logistic regression was applied to analyze the inhibition rate of ACM, MPNS and MPNS-ACM in the in vitro study.
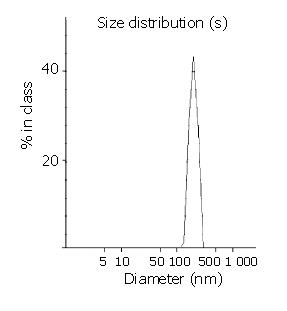
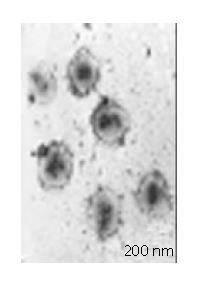
The average particle size was 210 nm and the size distribution range was 100-400 nm with the most frequent size around 210 nm (Figure 1). A typical core-shell structure is shown under TEM (Figure 2), it indicated black Fe3O4 was surrounded by white polymer. SEM photograph shows uniform sphere. The drug content of MPNS-ACM was 12.0%.
The tumor mass and volume were significantly decreased in both ACM group and MPNS-ACM group than control group at the end of the therapy (P < 0.05). The anti-tumor activity of high dosage of MPNS-ACM was higher than those of the other agents (Table 1).
The TIR of ACM was 22.63%. The tumor mass of ACM group on the eleventh day (1.06 ± 0.27 g) was much lower than that of control group (1.37 ± 0.21 g) (P < 0.05).
The TIR of high dosage of MPNS-ACM was 52.55%. The tumor mass on the eleventh day (0.65 ± 0.26 g) was much lower than those of the same dosage of ACM group, low dosage of MPNS-ACM group (0.95 ± 0.15 g), MPNS carrier group (1.23 ± 0.25 g) and control group.
The TIR of low dosage of MPNS-ACM (1.6 mg/kg bm) group was 30.66%, which was higher than that of ACM group (8 mg/kg bm), but there was no statistical difference as to the tumor mass between the two groups (P > 0.05). The tumor weight of low dosage of MPNS-ACM group was lower than those of MPNS group, and control group (P < 0.05).
The TIR of MPNS carrier was 10.22%. The tumor weight (1.23 ± 0.25 g) was similar to that of control group (1.37 ± 0.21, P > 0.05).
The white blood cell counts, serum ALT and creatine of the mice in all the groups were similar (P < 0.05) (Table 2). The number of CFU-GM of ACM group was much lower than those of the other four groups (P < 0.001). The white blood cell counts in ACM group was lower than that in control group, yet the ones in MNPS carrier and MNPS-ACM groups were similar to that in control group (P > 0.05, Table 2).
| Group | White blood cells (×109/L) | CFU-GM number (/104) | ALT (U/L) | Creatine (μmol/L) |
| ACM | 76.67 ± 17.32 | 74.75 ± 21.91b | 30.67 ± 16.51 | 12.17 ± 1.17 |
| High dosage of MPNS-ACM | 70.00 ± 8.74 | 107.83 ± 14.75 | 29.17 ± 14.36 | 12.50 ± 1.05 |
| Low dosage of MPNS-ACM | 74.67 ± 9.71 | 115.25 ± 12.53 | 23.83 ± 3.55 | 13.00 ± 2.19 |
| MPNS | 79.00 ± 11.23 | 117.50 ± 12.75 | 20.83 ± 4.71 | 12.00 ± 1.55 |
| Control | 74.83 ± 6.08 | 117.00 ± 12.48 | 37.83 ± 35.13 | 12.67 ± 1.51 |
Selective targeting agents are the trend of antineoplastic chemotherapy. However the production of the biodegradable agents of proper size, high targeting ability and good bioconsistency is an ongoing challenge. Small-sized magnetic particles(< 400 nm) can be extravasated into the tumor interstitium and retained there, owing to the enhanced permeability and retention effect of the tumor[24]. Polymer carriers encapsulated with magnetite are difficult to prepare because of the different solubility of magnetite and polymers. Here, a kind of modified superparamagnetic iron oxide particles was introduced to prepare the magnetic targeting agents and the particle size could be controlled to 210 nm also, which was very important for the tolerance and efficacy of the agents.
There were two steps for preparation of MPNS-ACM: the preparation and modification of superparamagnetic iron oxide nanoparticles and the synthesis of magnetic polymer loaded with drug. Chemical coprecipitation was applied to synthesize the iron oxide nanoparticles. After modification with acid, well-suspended and stable magnetite fluid was successfully made. It can be stored as suspension for over one year at room temperature. The magnetite can be localized under magnetic field and redispersed when the magnetic force disappears. Interfacial polymerization was applied to the second step where the biodegradable macromolecular material butylcyanoacrylate reacted at the interface between oil and water. Magnetic nanoparticles and fat soluble ACM were encapsulated during the polymerization. The encapsulated ACM was more stable than the one by attachment. The lyophilization agent can be stored long-term at room temperature. After complete ultrasonication, the nanoparticles intravenously administered could locate at the tumor by magnetic force. ACM was slowly released to produce high efficacy and low toxicity with the degradation of ploymer.
The results showed that the anti-tumor effect of MPNS-ACM in vitro without magnetic field was similar to that of MPNS carrier group (considering the drug content was 12% approximately), yet the anti-tumor test in vivo showed higher inhibitory efficacy of the magnetic carrier encapsulated with ACM on the gastric cancer model under magnetic field, which was based on the high targeting capacity of the system. TIR of targeted agent was higher than that of five-fold dosage of non-targeted drug. On the other hand, no toxicity to marrow, liver function and kidney function was found from targeted agents. The results show the high therapeutic efficacy on the tumor and the low toxicity to other organs of the magnetic targeted drug delivery system. It is a kind of safe chemotherapeutic agent.
Due to the difference between fat soluble drugs and water soluble drugs, different methods have been applied to encapsulate the drugs to the biopolymer carrier system with or without magnetite. The attempt to load ACM in to the carrier benefits the studies on other drugs including fat soluble drugs and water soluble drugs.
In conclusion, the magnetic targeted chemotherapy using MPNS-ACM has better tumor targeting, therapeutic efficacy and lower toxicity.
We are grateful to Ming Yao and Shi-Ming Fan of Shanghai Cancer Institute for their kind help with the animal experiments.
Edited by Chen WW Proofread by Zhu LH and Xu FM
| 1. | Gupta PK, Hung CT. Magnetically controlled targeted micro-carrier systems. Life Sci. 1989;44:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 105] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Widder KJ, Morris RM, Poore GA, Howard DP, Senyei AE. Selective targeting of magnetic albumin microspheres containing low-dose doxorubicin: total remission in Yoshida sarcoma-bearing rats. Eur J Cancer Clin Oncol. 1983;19:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Rudge S, Peterson C, Vessely C, Koda J, Stevens S, Catterall L. Adsorption and desorption of chemotherapeutic drugs from a magnetically targeted carrier (MTC). J Control Release. 2001;74:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | King ME, Kinney AY. Tissue adhesives: a new method of wound repair. Nurse Pract. 1999;24:66, 69-70, 73-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | de Verdière AC, Dubernet C, Némati F, Soma E, Appel M, Ferté J, Bernard S, Puisieux F, Couvreur P. Reversion of multidrug resistance with polyalkylcyanoacrylate nanoparticles: towards a mechanism of action. Br J Cancer. 1997;76:198-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 106] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Zhang ZR, He Q. Study on liver targeting and hepatocytes permeable valaciclovir polybutylcyanoacrylate nanoparticles. World J Gastroenterol. 1999;5:330-333. [PubMed] |
| 7. | Ravi Kumar MN. Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci. 2000;3:234-258. [PubMed] |
| 8. | Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2499] [Cited by in RCA: 2080] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 9. | Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19:99-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Kattan J, Droz JP, Couvreur P, Marino JP, Boutan-Laroze A, Rougier P, Brault P, Vranckx H, Grognet JM, Morge X. Phase I clinical trial and pharmacokinetic evaluation of doxorubicin carried by polyisohexylcyanoacrylate nanoparticles. Invest New Drugs. 1992;10:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Stella B, Arpicco S, Peracchia MT, Desmaële D, Hoebeke J, Renoir M, D'Angelo J, Cattel L, Couvreur P. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89:1452-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Brigger I, Chaminade P, Marsaud V, Appel M, Besnard M, Gurny R, Renoir M, Couvreur P. Tamoxifen encapsulation within polyethylene glycol-coated nanospheres. A new antiestrogen formulation. Int J Pharm. 2001;214:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Calvo P, Gouritin B, Chacun H, Desmaële D, D'Angelo J, Noel JP, Georgin D, Fattal E, Andreux JP, Couvreur P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 299] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Li YP, Pei YY, Zhou ZH, Zhang XY, Gu ZH, Ding J, Zhou JJ, Gao XJ, Zhu JH. Stealth polycyanoacrylate nanoparticles as tumor necrosis factor-alpha carriers: pharmacokinetics and anti-tumor effects. Biol Pharm Bull. 2001;24:662-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Vauthier C, Dubernet C, Fattal E, Pinto-Alphandary H, Couvreur P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliv Rev. 2003;55:519-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 16. | Skidan IN, Gel'perina SE, Severin SE, Guliaev AE. [Enhanced activity of rifampicin loaded with polybutyl cyanoacrylate nanoparticles in relation to intracellularly localized bacteria]. Antibiot Khimioter. 2003;48:23-26. [PubMed] |
| 17. | Zhang ZR, He Q, Liao GT, Bai SH. Study on the anticarcinogenic effect and acute toxicity of liver-targeting mitoxantrone nanoparticles. World J Gastroenterol. 1999;5:511-514. [PubMed] |
| 18. | Zhang Q, Shen Z, Nagai T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm. 2001;218:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Deng Y, Wang L, Yang W, Fu S, Elaissari A. Preparation of magnetic polymeric particles via inverse microemulsion polymer-ization process. J Magnetism Magnetic Materials. 2003;257:69-78. [DOI] [Full Text] |
| 20. | Kreuter J. Evaluation of nanoparticles as drug-delivery systems I: perparation methods. Pharm Acta Helv. 1983;58:196-209. |
| 21. | Sommerfeld P, Schroeder U, Sabel BA. Long-term stability of PBCA nanoparticle suspensions suggests clinical usefulness. Int J Pharm. 1997;155:201-207. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349-3356. [PubMed] |
| 23. | Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387-6392. [PubMed] |
| 24. | Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752-3756. [PubMed] |