Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1971
Revised: February 24, 2004
Accepted: March 4, 2004
Published online: July 1, 2004
AIM: To observe the anti-cancer effects of COX-2 inhibitors and investigate the relationship between COX-2 inhibitors and angiogenesis, infiltration or metastasis in SGC7901 cancer xenografts.
METHODS: Thirty athymic mice xenograft models with human stomach cancer cell SGC7901 were established and divided randomly into 3 groups of 10 each. Sulindac, one non-specific COX inhibitor belonging to non-steroidal anti-inflammatory drugs (a series of COX inhibitors known as NSAIDs) and celecoxib, one selective COX-2 inhibitor (known as SCIs) were orally administered to mice of treatment groups. Immunohistochemistry was used to examine the expression of PCNA, CD44v6 and microvessel density (MVD). Apoptosis was detected by using TUNEL assay.
RESULTS: Tumors in sulindac and celecoxib groups were significantly smaller than those in control group from the second week after drug administration (P < 0.01). In treatment group, the cell proliferation index was lower (P < 0.05) and apoptosis index was higher (P < 0.05) than those in control groups. Compared with the controls, microvessel density was reduced (P < 0.01) and expression of CD44v6 on tumor cells was weakened (P < 0.05) in treatment groups.
CONCLUSION: COX-2 inhibitors have anticancer effects on gastric cancer. They play important roles in angiogenesis and infiltration or metastasis of stomach carcinoma. The anticancer effects of COX-2 inhibitors may include inducing apoptosis, suppressing proliferation, reducing angiogenesis and weakening invasiveness.
- Citation: Fu SL, Wu YL, Zhang YP, Qiao MM, Chen Y. Anti-cancer effects of COX-2 inhibitors and their correlation with angiogenesis and invasion in gastric cancer. World J Gastroenterol 2004; 10(13): 1971-1974
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1971
Gastric cancer is one of the commonest malignancies of human beings. The incidence of gastric cancer is typically high in China and as a result, more than 170000 people die of it each year. It has important significance if certain drugs are found to lower its incidence or prevent it.
Chemoprevention of NSAIDs against colorectal cancer has been observed for long[1]. Since cyclooxygenase-2 (COX-2), one of the isoenzymes catalyzing the production of prostaglandins, was discovered in early 1990s[2], its gene construction, biochemical property and biological role have been understood step by step. The discovery of COX-2 has enlightened people to pay more attention to its relation with neoplasm. More and more selective COX-2 inhibitors (SCIs) have been found out, further facilitating the cognition to COX-2[3]. Although the roles COX-2 inhibitors play in various cancers and their mechanisms are being widely studied recently, few people have gone deep into in vivo experiments[4]. Based on in vitro cytologic experiments[5-7], this study went further into in vivo experiments so as to clarify the anti-cancer mechanisms of COX-2 inhibitors.
Human moderately differentiated gastric cancer cell line SGC7901 was cultured in RPMI 1640 medium at 37 °C in a humidified box (Hareus) with 50 mL/L CO2 in our laboratory. When cells were amplified to a certain amount, they were dissociated, collected and suspended in PBS at a density of 5 × 107/mL.
Thirty male athymic mice (BALB/c nu/nu, 6 wk old, 17-20 g) were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences. Mice were maintained under specific pathogen-free conditions (Micro-FLO positive air supply rodent cage system) and fed with sterilized food and autoclaved water. Experiments were started after 3 d of acclimatization.
Gum arabic (50 mg/kg) was dissolved in sterilized water at a concentration of 10 mg/mL. Sulindac (8 mg/kg; Sigma inc.) and celecoxib (10 mg/kg) were agitated and suspended with gum arabic (50 mg/kg) in water at a same concentration, respectively, by using a homogenizer.
Each mouse was inoculated with a subcutaneous injection of SGC7901 cells (5 × 106 in 0.1 mL PBS) into the right forelimb after weighed individually. Then these 30 mice were randomized into control, sulindac, and celecoxib groups. From the same day, the mice were orally administered different agents once daily (0.1mL; according to mouse weight of 20 g): the controls with gum arabic, the sulindac group with sulindac, and the celecoxib group with celecoxib. Mice’s diet, activity, stool, urine, and tumor growth were observed daily and shortest and longest diameters of xenografts were measured weekly. The tumor volume was deduced according to the formula[8]: volume (mm3) = (the shortest diameter)2× (the longest diameter)/2. Both body weight and tumor size of each mouse were measured again before they were killed by cervical dislocation on the 32 nd day. All tumors were dissected from the body and weighed, then divided along the longest diameter. Halves of the specimens were frozen in liquid nitrogen while the other halves were fixed in 40 g/L phosphate-buffered formaldehyde.
The formalin-fixed tissues were embedded in paraffin, and sectioned at a thickness of 4 μm. The sections were deparaffinized and hydrated gradually, and examined by histology of HE staining, immunohistochemistry, and TUNEL technique respectively. EnVision kits, the reagents of immunohistochemical assay, were purchased from GeneTech Co. Tests were performed according to the two step procedure. After incubated with 3% H2O2 for 10 min at room temperature and unmasked antigens by heat treatment, sections were covered with animal serum for 20 min. Specimens were then incubated with primary antibodies PCNA (PC10; 1/100; Santa Cruz), CD44v6 (ZM-0052; Beijing Zhongshan), or CD34 (BD) at 4 °C over night and further treated with EnVision kits for 30 min at room temperature. They were visualized by diaminobenzidin (DAB) and counter-stained by hematoxylin. TBS took the place of primary antibodies as a negative control. Sections were observed under microscope after mounted. The results of staining were analyzed and evaluated with American Image-Pro Plus software. The percentage of positive cells with PCNA staining in five 400 × sights was counted as proliferation index (PI). The average of vessels with CD34 staining in three hot regions was calculated as MVD.
The reagent kit for apoptosis detection, TdT-FragEL DNA fragmentation detection kit was bought from ONCOGENE. Test procedures consisting of the following sections were provided in the brochure of the kit. The specimens were deparaffinized and hydrated gradually, and rinsed with 1 × TBS, then incubated with proteinase K (20 μg/mL in 10 mmol/L Tris-HCl) for 20 min. After immersed in 30 mL/L H2O2 at room temperature for 5 min and in TdT labeling reaction mixture at 37 °C for 1.5 h, specimens were covered with 1 × conjugate for 30 min, visualized by DAB and counter-stained by hematoxylin afterwards. TBS took the place of primary antibodies as a negative control. After mounted, sections were observed under microscope. The results of staining were analyzed and evaluated with American Image-Pro Plus software. The percentage of positive cells with TUNEL staining in five 400 × sights served as apoptosis index (AI).
Data were analyzed by software of SAS 6.12 and shown in a default form of mean ± SD.
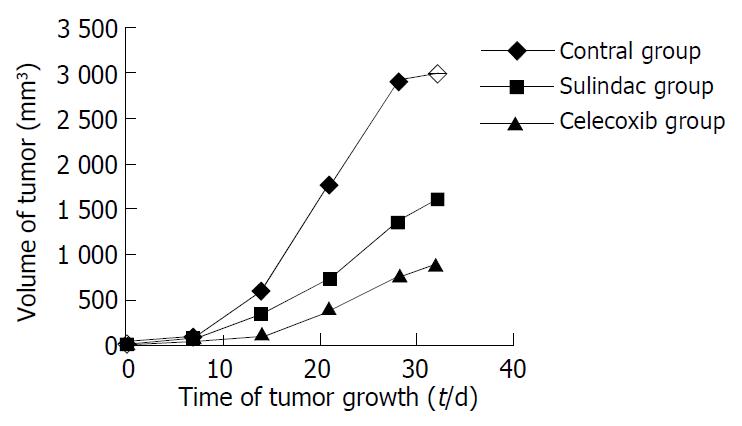
During the experiment, the growth, diet, activity, etc. of mice were carefully observed, no hematuresis and hematochezia were shown during experiment. Two mice died accidentally in the process however, which might be caused by the operation of enema clyster. The body weight among groups was not significantly different, nor did the weight change during the experiment. The growth of xenografts in treatment groups was significantly suppressed compared with the controls, but there was no difference between two treatment groups (Figure 1).
Difference of tumor growth in different groups was shown from the second week (Table 1).
| Group | Date (d) | ||||
| 7 | 14 | 21 | 28 | 32 | |
| Contro l | 45.2 ± 35.5 | 609 ± 289 | 1779 ± 366 | 2920 ± 776 | 2984 ± 589 |
| Sulindac | 78 ± 137 | 351.5 ± 227.0 | 723 ± 514 | 1370 ± 832 | 1590 ± 1009 |
| Celecoxib | 19.5 ± 14.8 | 108 ± 105 | 408 ± 390 | 788 ± 701 | 891 ± 764 |
| P | 0.30 | 0.0002 | 0.0001 | 0.0001 | 0.0001 |
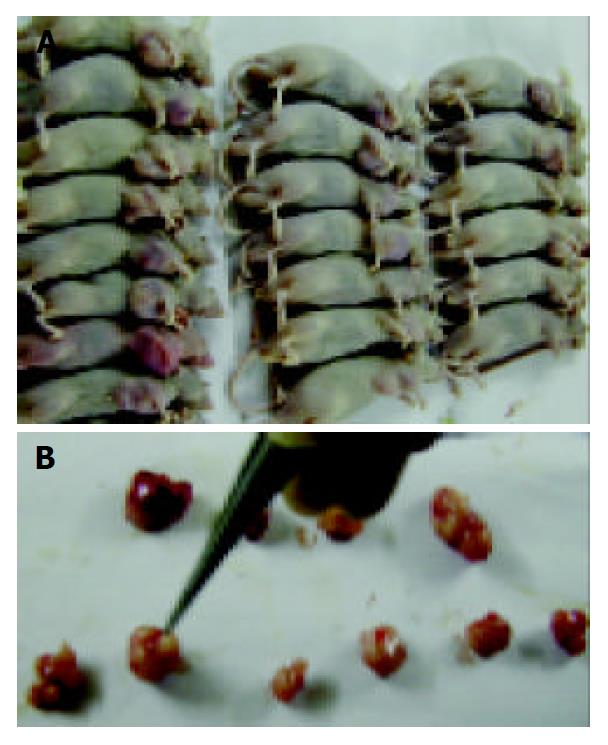
In each group, five mice out of ten were picked out randomly and dissected with no obvious erosion, bleeding, or ulcer of stomach and no neoplasm metastasis.
Xenograft took on an appearance of big globular neoplasia or cluster of several small neoplastic nods (Figure 2). Necrosis could be seen comparatively common in cut of tumors, and the size of necrotic area seemed to be related to the volume of tumor. With regard to HE-stained sections, deeply stained tumor cells with big nuclei were arranged tightly with no cavum structure found under microscope, which coincided with the characteristics of tumor histology. Tumor cells among all groups showed no difference in morphology, while necrosis could be commonly seen under microscope.
PI of control group was significantly higher than that of sulindac group and celecoxib group (P < 0.05), but the difference was not notable between two treatment groups. AI in two treatment groups was higher than that in the control group (P < 0.05), while there was also no difference between the two groups. The AI/PI value was calculated and compared among all groups. Consequently, it was apparently larger in two treatment groups (P < 0.01), however no difference was shown between them.
Immunohistochemical staining of CD34 revealed that celecoxib and sulindac could suppress angiogenesis of SGC7901 xenografts. MVD in sulindac and celecoxib groups was apparently lower than that in the control (P < 0.01). Although it was lower in celecoxib group, the difference is not notable.
Membranes of tumor cells were stained brown by CD44v6 staining. By analysis of staining intensity and quantity of positive cells with Image-Pro Plus software, the expression of CD44v6 was markedly weakened by the treatment with sulindac and celecoxib (P < 0.05), but there was no apparent difference between sulindac and celecoxib groups.
COX-2 was successfully cloned and its structure has been clearly recognized more than ten years before[9]. It is an inducible isoenzyme that catalyzes production of a series of prostaglandins. Participating in inflammatory reaction, COX-2 is expressed by a variety of tumor cells and correlated to tumorigenesis. Several researches have revealed the prophylactic effect of NSAIDs on colorectal carcinoma and their therapeutic effect on colon polyps[10]. Here the mechanism of NSAIDs is considered as inhibiting COX-2. Non-specific COX inhibitors inhibit COX-1 at the same time, which may cause fatal side effects. As a result, they are not so ideal in long-term application for preventing tumorigenesis. Lately developed selective COX-2 inhibitors shed light on chemoprevention of neoplasms. Nevertheless a series of researches have to be carried out to confirm its effectiveness, reliability and virtues before extensive clinical application.
The expression of COX-2 also existed in gastric cancer while the positive rate might reach 61.4%[11]. We have shown that human moderately differentiated gastric cancer cell SGC7901 can express COX-2. Its growth was suppressed in vitro after the treatment of sulindac, both proliferation and apoptosis were affected[5]. This time we inoculated athymic mice with SGC7901 to observe the effects of sulindac and celecoxib, a clinically applied selective COX-2 inhibitor, on in vivo tumor by establishing animal models of gastric cancer. The results showed that both drugs had a notable inhibition on gastric cancer growth. Although the effect of celecoxib was better than of sulindac, no statistical difference was shown.
To explore the anticancer mechanisms of COX-2 inhibitors, we evaluated the influence of two drugs on tumor cell proliferation and apoptosis in xenografts by immunohistochemistry, which verified the results of in vitro researches. Administration of both sulindac and celecoxib increased apoptosis of cancer cells in vivo. The AI/PI, a value reflecting cytokinetics, showed a more significant difference.
The growth of tumor cells depends on nutrition supply, which largely relies on angiogenesis. Ischemia can induce tumor cell apoptosis, speeding up necrosis and cell extinction. Many researches verified the relation between COX-2 and angiogenesis[12] and the inhibition effects of NSAIDs on blood vessel endothelial cells[13]. We observed that sulindac and celecoxib obviously decreased the blood vessel quantity of xenografts and reduced the MVD compared to that of the control group. COX-2 inhibitors realized their anti-cancer effects by repressing the expression of anti-apoptosis gene Bcl-2[14] and reducing angiogenesis in stomach carcinoma, thereby impairing the nutrition supply of the tumor, further inhibiting proliferation and inducing apoptosis of gastric cancer cells. Our results were similar to those of Sawaoka et al[15].
Some studies suggested the expression of COX-2 was correlated to the clinicopathologic characteristics of gastric cancer, such as infiltration, lymphatic or hematogenous metastasis, prognosis, etc[16]. As a cell surface adhesive molecule, CD44 was the receptor of hyaluronic acid and involved in cell-to-cell and cell-to-matrix interactions. Especially, the expression of its spliced variant 6 was closely correlated to cell movement, carcinogenesis, progress, incursion and metastasis of gastric carcinoma[17]. In this study we evaluated COX-2 inhibitors’ influence on the CD44v6 expression by using animal models, finding the positive cells of expressing CD44v6 (pigmented in membrane) often existed in the periphery of tumors with a tendency to surround blood vessels. The positivity of CD44v6 staining was strong in the control group and significantly weakened in two medication groups, which demonstrating that COX-2 inhibitors play a role in depressing invasiveness and reducing metastasis of gastric cancer, which could be one of their anti-cancer effects. No obvious metastasis was found by rough anatomy of mice in our study however. It requires improved experiment design.
In brief, the mechanisms of COX-2 inhibitors resisting the growth of gastric cancer might include suppressing cell proliferation, inducing apoptosis, reducing angiogenesis and weakening invasiveness. But selective COX-2 inhibitors were not observed obviously more effective than non-specific COX inhibitors. The former did not show any advantage in side effects either, such as gastrorrhagia, ulceration, and so on, which may be relevant to experiment animal model and short experiment duration. Further studies are required. This study also showed comparatively more necrosis of tumors, but its correlation to drug administration was unclear . In comparison, Japanese researchers discovered no relation between drug treatment of indomethacin or NS398 in MKN45 cell xenografts[15].
This is the first part of a serial study, and we will verify the results afterwards using Western blotting, RT-PCR, etc.
Edited by Wang XL and Chen WW Proofread by Xu FM
| 1. | Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48:4399-4404. [PubMed] |
| 2. | Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 1991;88:2692-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1144] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 3. | Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-412. [PubMed] |
| 4. | Hahm KB, Lim HY, Sohn S, Kwon HJ, Lee KM, Lee JS, Surh YJ, Kim YB, Joo HJ, Kim WS. In vitro evidence of the role of COX-2 in attenuating gastric inflammation and promoting gastric carcinogenesis. J Environ Pathol Toxicol Oncol. 2002;21:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997-1002. |
| 6. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [PubMed] |
| 7. | Li JY, Wang XZ, Chen FL, Yu JP, Luo HS. Nimesulide inhibits proliferation via induction of apoptosis and cell cycle arrest in human gastric adenocarcinoma cell line. World J Gastroenterol. 2003;9:915-920. [PubMed] |
| 8. | Ovejera AA, Houchens DP, Barker AD. Chemotherapy of human tumor xenografts in genetically athymic mice. Ann Clin Lab Sci. 1978;8:50-56. [PubMed] |
| 9. | Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384-7388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1043] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635-639. [PubMed] |
| 11. | Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1644] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 13. | Sun B, Wu YL, Zhang XJ, He HY, Wang SN, Qiao MM. Growth inhibition of Sulindac and Indomethacin on human umbilical vein endothelial cells ECV304. Zhongliu. 2003;23:370-372. |
| 14. | Sun B, Wu YL, Wang SN, Zhang XJ, He HY, Qiao MM, Zhang J. The effects of sulindac on induction of apoptosis and expression of cyclooxygenase-2 and Bcl-2 in human hepatocellular carcinoma cells. Zhonghua Xiaohua Zazhi. 2002;22:338-340. |
| 15. | Sawaoka H, Tsuji S, Tsujii M, Gunawan ES, Sasaki Y, Kawano S, Hori M. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab Invest. 1999;79:1469-1477. [PubMed] |
| 16. | Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 17. | Joo M, Lee HK, Kang YK. Expression of E-cadherin, beta-catenin, CD44s and CD44v6 in gastric adenocarcinoma: relationship with lymph node metastasis. Anticancer Res. 2003;23:1581-1588. [PubMed] |