Published online Jul 1, 2004. doi: 10.3748/wjg.v10.i13.1939
Revised: September 28, 2003
Accepted: October 7, 2003
Published online: July 1, 2004
AIM: To assess the value of computed tomography during arterial portography (CTAP) in portal vein-vena cava shunt, and analysis of the episode risk in encephalopathy.
METHODS: Twenty-nine patients with portal-systemic encephalopathy due to portal hypertension were classified by West Haven method into grade I (29 cases), grade II (16 cases), grade III (10 cases), grade IV ( 4 cases). All the patients were scanned by spiral-CT. Plane scans, artery phase and portal vein phase enhancement scans were performed, and the source images were thinly reconstructed to 1.25 mm. We reconstructed the celiac trunk, portal vein, inferior vena cava and their branches and subjected them to three-dimensional vessel analysis by volume rendering (VR) technique and multiplanar volume reconstruction (MPVR) technique. The blood vessel reconstruction technique was used to evaluate the scope and extent of portal vein-vena cava shunt, portal vein emboli and the fistula of hepatic artery-portal vein. The relationship between the episode risk of portal-systemic encephalopathy and the scope and extent of portal vein-vena cava shunt, portal vein emboli and fistula of hepatic artery-portal vein was studied.
RESULTS: The three-dimensional vessel reconstruction technique of spiral-CT could display celiac trunk, portal vein, inferior vena cava and their branches at any planes and angles and the scope and extent of portal vein-vena cava shunt, portal vein emboli and the fistula of hepatic artery-portal vein. In twenty-nine patients with portal-systemic encephalopathy, grade I accounted for 89.7% esophageal varices, 86.2% paragastric varices; grade II accounted for 68.75% cirsomphalos, 56.25% paraesophageal varices, 62.5% retroperitoneal varices and 81.25% dilated azygos vein; grade III accounted for 80% cirsomphalos, 60% paraesophageal varices, 70% retroperitoneal varices, 90% dilated azygos vein, and part of the patients in grades II and III had portal vein emboli and fistula of hepatic artery-portal vein; grade IV accounted for 75% dilated left renal vein, 50% paragallbladder varices, all the patients had fistula of hepatic artery-portal vein.
CONCLUSION: The three-dimensional vessel reconstruction technique of spiral-CT can clearly display celiac trunk, portal vein, inferior vena cava and their branches at any planes and angles and the scope and extent of portal vein-vena cava shunt. The technique is valuable for evaluating the episode risk in portal-systemic encephalopathy.
- Citation: Chu Q, Li Z, Zhang SM, Hu DY, Xiao M. Relationship between encephalopathy and portal vein-vena cava shunt: Value of computed tomography during arterial portography. World J Gastroenterol 2004; 10(13): 1939-1942
- URL: https://www.wjgnet.com/1007-9327/full/v10/i13/1939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i13.1939
Portal-systemic encephalopathy is a kind of syndrome caused by portal hypertension and the construction of lateral branches, leading to a series of metabolic disorders, and is related to many neuron conduction mechanisms[1]. The main clinical manifestation is coma or the alteration of consciousness and behaviors. The syndrome has a close relationship with the construction of portal vein lateral branches. The three-dimensional vessel reconstruction technique of spiral-CT is the best method to display the vascular system[2]. Using this technique, we detected the scope and degree of portal vein-vena cava shunt, analyzed the risk of portal-systemic encephalopathy, and the relationship between them. This is of importance for doctors to make appropriate therapy plan and predict the disease’s prognosis.
From April to October 2003, 480 patients were performed spiral-CT because of liver or portal vein diseases. Among them, 59 patients had portal-systemic encephalopathy, including 44 males and 15 females. They aged from 34 to 69 years, averaging 48.9 years. All the patients were diagnosed by clinical symptoms, laboratory examination and radiologic findings. They were classified by West Haven method into grade I (29 cases), grade II (16 cases), grade III (10 cases), grade IV (4 cases).
Based on West Haven method, encephalopathy was divided into 4 grades[3]. Grade I had absence of normal consciousness, euphoria or anxious, shortening of attention time, and other damage manifestations. Grade II had somnolence or indifference, mild disorder of time or space, mild change of personality, unsuitable behaviors, damage of subtraction. Grade III had lethargy, even half coma, reactions to word stimulation, confusion, obvious orientation disorder. Grade IV had coma (The patient had no reaction to word stimulation or harmful stimulation).
Lightspeed spiral-CT (GE Corporation, USA) performed high-speed scan. The parameters[4] were 120 KV, 250 mA, slice thickness 10 mm, pitch 1.375:1, and matrix 512 × 512. We performed plane scan at first, and then enhancement scan. The dosage of Ultravist 300 (62.3 mg/mL) was 2 mL/kg, and the injection speed was 3.5 mL/s. The injection was executed by a hyperbaric injection syringe in elbow vein by bolus injection. We performed the artery phase and portal vein phase enhancement scans after the injection[5].
Double-phase enhancement 10-mm thick images were reconstructed in CT[6], and the reconstructed slices were 1.25-mm thick. Then the images were pushed to the workstation. The three-dimensional blood vessel reconstruction was performed by ADW4.0 software. The reconstructed celiac trunk, portal vein, inferior vena cava and their branches were subjected to three-dimensional vessel analysis by volume rendering (VR) technique[7,8] and multiplanar volume reconstruction (MPVR) technique[9]. The VR technique could utilize any axis as basic axis and carry out 360-degree rotation so that we could observe the vessels in any degree[10]. The MPVR technique could perform any slice cross-sectional analysis[11]. We evaluated the scope and degree of portal vein-vena cava shunt, portal vein emboli and the fistula of hepatic artery-portal vein with the aid of source images and reconstructed images[12], and analyzed the relationship between the above-mentioned changes and the risk of portal-systemic encephalopathy[13,14].
Referring to Ishikawa vessel classification method and considering the patients’ condition, we classified the portal vein-vena cava shunt into nine types, normally cirsomphalos, paragallbladder varices, lienorenal vein collateral branch opening, retroperitoneal varices, esophageal varices, paraesophageal varices, dilated azygos vein, paragastric varices and dilated left renal vein. At the same time, we observed whether there were portal vein emboli and fistula of hepatic artery-portal vein.
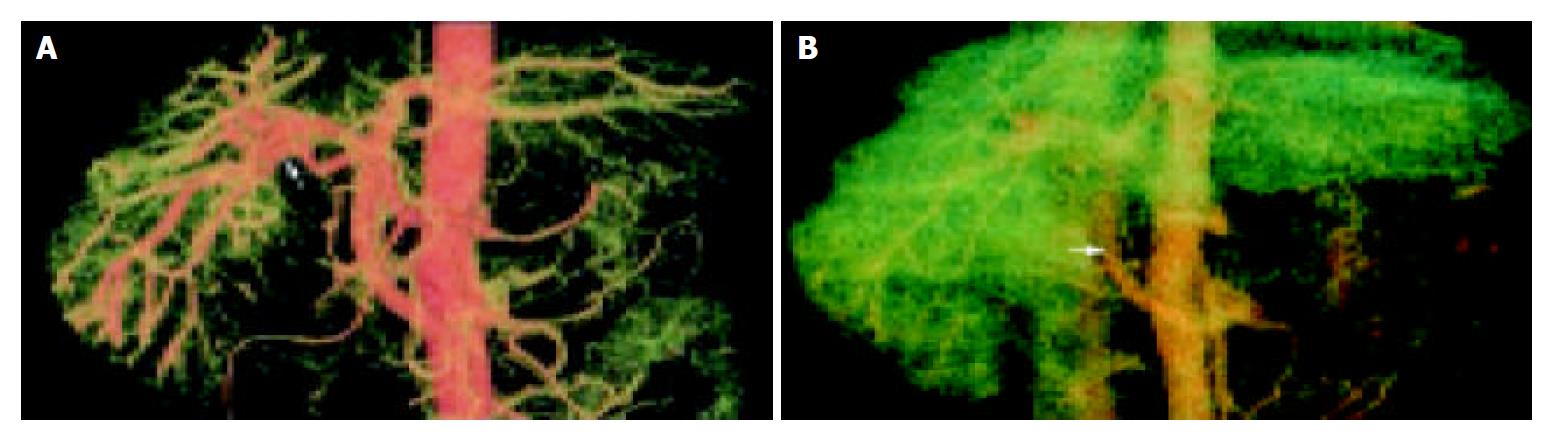
The VR technique could display the 3-dimensional vascular structures of celiac trunk, hepatic artery, superior mesenteric artery and their branches at any angle in artery phase. We could analyze the relationship between the arteries and portal vein. The VR technique could display artery-portal vein fistula very well. In the artery phase, the portal vein was visualized early, and the blood in portal vein was seen refluxed (Figure 1A). In portal vein phase, the portal vein was very slightly developed and portal vein-vena cava shunt emerged (Figure 1B).
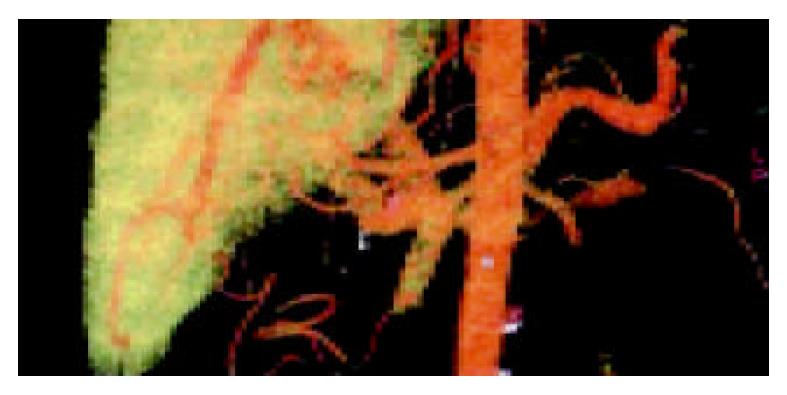
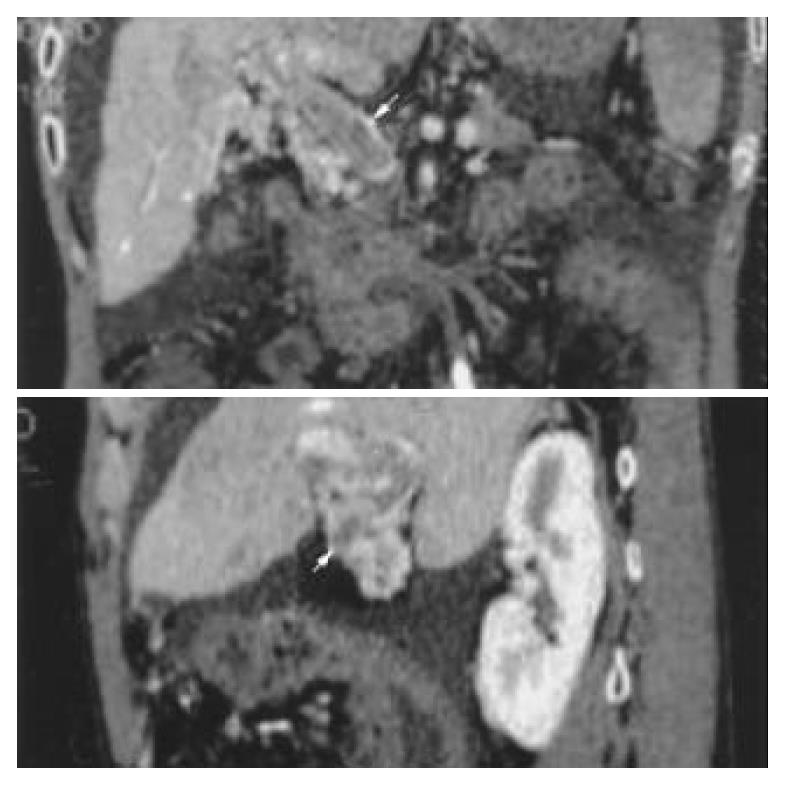
In the portal vein phase, the VR technique could show the 3-dimensional vascular structures of portal vein and its branches at any angle. The enlarged portal vein and a non-flow region in the trunk could be seen (Figure 2). An image of portal vein was reconstructed by MPVR, and the emboli’s scope and size could be displayed at many angles (Figure 3).
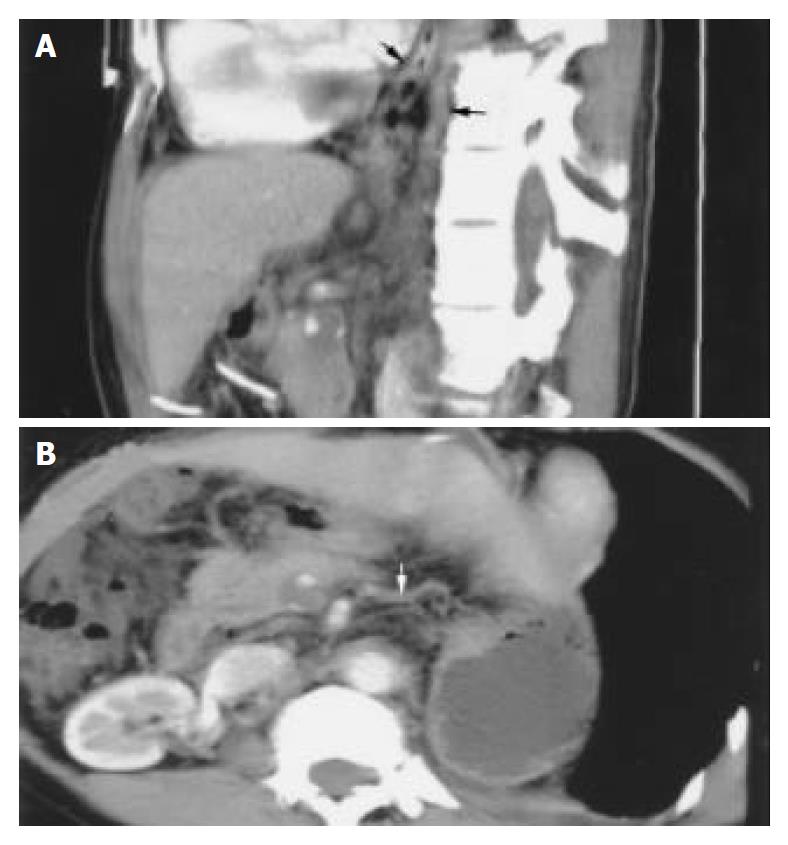
The MPVR technique could perform any cross-section analysis. Combining the source images, we evaluated the scope and degree of portal vein-vena cava shunt, portal vein emboli and fistula of hepatic artery-portal vein on MPVR images (Figure 4A, B).
Referring to Ishikawa vessel classification method, the patients were classified into 4 grades by West Haven method: grade I, 29 cases; grade II, 16 cases; grade III, 10 cases; grade IV, 4 cases. The main portal vein disorders are listed in Table 1.
| Grade I | Grade II | Grade III | Grade IV | |
| n (%) | n (%) | n (%) | n (%) | |
| Esophageal varices | 26 (89.7) | 9 (56.25) | 8 (80) | 4 (100) |
| Paragastric varices | 25 (86.2) | 11 (68.75) | 7 (70) | 3 (75) |
| Cirsomphalos | 12 (41.3) | 11 (68.75) | 8 (80) | 4 (100) |
| Retroperitoneal varices | 11 (37.9) | 9 (56.25) | 6 (60) | 4 (100) |
| Paraesophageal varices | 13 (44.8) | 10 (62.5) | 7 (70) | 3 (75) |
| Dilated azygos vein | 6 (20.1) | 13 (81.25) | 9 (90) | 2 (50) |
| Dilated left renal vein | 2 (6.9) | 1 (6.25) | 1 (10) | 3 (75) |
| Paragallbladder varices | 0 (0) | 1 (6.25) | 1 (10) | 2 (50) |
| Hepatic artery-portal vein fistula | 0 (0) | 3 (18.75) | 6 (60) | 4 (100) |
| Portal vein emboli | 2 (6.9) | 8 (50) | 7 (70) | 3 (75) |
As it shows in this table, esophageal varices and paragastric varices were common in grade I. Besides the varices in grade I patients, the patients in grades II and III had cirsomphalos, paraesophageal varices, retroperitoneal varices and dilated azygos vein, and some of them had portal vein emboli and hepatic artery-portal vein fistula. Among the patients in grade IV, dilated left renal vein and paragallbladder varices were usual, and all patients had hepatic artery-portal vein fistula.
Encephalopathy is a nervous system syndrome, originating from chronic liver disease and hepatic cirrhosis. It is associated with portal hypertension, hepatic artery-portal vein fistula, portal vein emboli, hyperammonemia and other metabolic disorders. It also has a close relationship with hypertension, portal vein-vena cava shunt and lateral branch formation. The three-dimensional vessel reconstruction technique of spiral-CT can display portal vein, its branches, the scope and degree of portal vein-vena cava shunt. The technique is valuable for evaluating the risk of encephalopathy.
Spiral-CT double-phase (artery phase, portal vein phase) scan and the reconstruction technique could display portal vein-vena cava shunt, hepatic artery-portal vein fistula and portal vein emboli[15]. Computed tomography during arterial portography (CTAP) was one of the best ways to show portal vein disorders[16]. Sixteen-slice spiral-CT had unique superiority for portal vein pictures[17]. To evaluate hepatic artery-portal vein fistula, early visualization of portal vein, enlargement of portal vein and blood of portal vein reflux were the main characteristics[18,19]. The double-phase images of volume rendering technique could not only display the position and size of fistulae, but also the diameter of portal vein and the extent of portal vein reflux. Reflux and fistulae would advance the portal hypertension, accelerate the portal vein-vena cava shunt, decrease the portal vein blood flow into the liver, and increase the opportunity for encephalopathy[20]. There was no hepatic artery-portal vein fistula in grade I patients (n = 29), only 18.75% patients had fistulae in grade II (Table 1). However, in grades III and IV patients, 60% and 100% patients had fistulae. We can draw a conclusion that hepatic artery-portal vein fistula increases the risk of encephalopathy, and fistulae would aggravate encephalopathy or indicate the risk of encephalopathy.
On the assessment of portal vein emboli, the VR images in portal vein phase could show the vein because the blood flow was hindered by emboli or the complete obstruction of the trunk. The portal vein embolus was possibly one of the markers that hint the deterioration of encephalopathy[21,22]. Perhaps portal vein embolus could hinder blood flow into the liver[23]. It was believed that encephalopathy would appear if the blood flow of portal vein was less than 692 mL/min[24]. The portal vein embolus would accelerate portal hypertension and induce brain disorder at the same time.
Portal vein-vena cava shunts include[25,26] esophageal varices, paragastric varices[27], paraesophageal varices, cirsomphalos, paraumbilical veins[28,29], retroperitoneal varices (Retzius venous plexus), dilated azygos vein, dilated spleno-renal vein and stomach-renal vein, even internal iliac vein communication[30]. CT and barium meal had the same role in diagnosing esophageal varices and paragastric varices. But CT was more valuable than barium meal and esophagoscope in diagnosing other varices[31,32]. Spiral-CT scan is a kind of volume scan, and its merits include high speed and short reconstruction time. An examination of a patient’s liver can be finished in a breathholding. VR images could display large lateral branch circulation clearly, but the small branches unclearly. The multiplanar volume reconstruction (MPVR) technique could perform any slice cross-sectional analysis of the vessels and disclose their relationship with the surrounding tissues (Figure 2, Figure 3), and esophageal varices, paragastric varices and azygos vein.
In a word, simple esophageal varices, paragastric varices and paraesophageal varices would not induce severe encephalopathy. However, the portal vein emboli, or hepatic artery-portal vein fistula, or wide portal vein-vena cava shunt, particularly the dilated left renal vein and paragallbladder varices, which may hint encephalopathy, would occur or deteriorate.
Not only would portal vein-vena cava shunt induce portal-systemic encephalopathy, but also the liver function of patients, metabolic status[33] and diseases outside the liver[34]played a role. The relationship between them is still unclear. More work needs to be done. However, the three-dimensional vessel reconstruction technique of spiral-CT can display the celiac trunk, portal vein, inferior vena cava and their branches at any plane and from any angle, and show the scope and degree of portal vein-vena cava shunt. The technique is valuable for evaluating the episode risk in portal-systemic encephalopathy, and helpful for TIPSS.
Edited by Wang XL and Zhu LH Proofread by Xu FM
| 1. | Butterworth RF. Hepatic encephalopathy: a neuropsychiatric disorder involving multiple neurotransmitter systems. Curr Opin Neurol. 2000;13:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Calculli L, Casadei R, Amore B, Albini Riccioli L, Minni F, Caputo M, Marrano D, Gavelli G. The usefulness of spiral Computed Tomography and colour-Doppler ultrasonography to predict portal-mesenteric trunk involvement in pancreatic cancer. Radiol Med. 2002;104:307-315. |
| 3. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1402] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 4. | Francis IR, Cohan RH, McNulty NJ, Platt JF, Korobkin M, Gebremariam A, Ragupathi KI. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. AJR Am J Roentgenol. 2003;180:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Matoba M, Yokota H, Yabuno K, Kuga G, Tinami H, Yamamoto I. [Evaluation of two injection protocols by time-density curves for possible application to hepatic dynamic and upper abdominal CT angiography in MDCT: high concentration (350 mgI/ml) with conventional volume (100 ml) vs. conventional concentration (300 mgI/ml) with larger volume (150 ml) and higher injection rate]. Nihon Igaku Hoshasen Gakkai Zasshi. 2003;63:98-102. [PubMed] |
| 6. | Jayakrishnan VK, White PM, Aitken D, Crane P, McMahon AD, Teasdale EM. Subtraction helical CT angiography of intra- and extracranial vessels: technical considerations and preliminary experience. AJNR Am J Neuroradiol. 2003;24:451-455. [PubMed] |
| 7. | Dudeck O, Hoffmann KT, Wieners G, Pech M, Knollmann F, Felix R. [Application of multislice detector spiral computed tomography to intracranial aneurysms: first clinical experience]. Radiologe. 2003;43:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Byun JH, Kim TK, Lee SS, Lee JK, Ha HK, Kim AY, Kim PN, Lee MG, Lee SG. Evaluation of the hepatic artery in potential donors for living donor liver transplantation by computed tomography angiography using multidetector-row computed tomography: comparison of volume rendering and maximum intensity projection techniques. J Comput Assist Tomogr. 2003;27:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Fröhner S, Wagner M, Schmitt R, Brunn J, Müller M, Christopoulos G, Coblenz G, Kerber S, Urbanski P. [Multi-slice CT of aortocoronary venous bypasses and mammary artery bypasses: evaluation of bypasses and their anastomoses]. Rontgenpraxis. 2002;54:163-173. [PubMed] |
| 10. | Fink C, Hallscheidt PJ, Hosch WP, Ott RC, Wiesel M, Kauffmann GW, Düx M. Preoperative evaluation of living renal donors: value of contrast-enhanced 3D magnetic resonance angiography and comparison of three rendering algorithms. Eur Radiol. 2003;13:794-801. [PubMed] |
| 11. | Kuiper JW, Geleijns J, Matheijssen NA, Teeuwisse W, Pattynama PM. Radiation exposure of multi-row detector spiral computed tomography of the pulmonary arteries: comparison with digital subtraction pulmonary angiography. Eur Radiol. 2003;13:1496-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | van Straten M, Venema HW, Streekstra GJ, Reekers JA, den Heeten GJ, Grimbergen CA. Removal of arterial wall calcifications in CT angiography by local subtraction. Med Phys. 2003;30:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kobayashi Y, Nakazawa J, Sakata M. Comparison of the depic-tion of pancreaticoduodenal arcades and dorsal pancreatic artery, using three-point scale with volume rendering (VR), maximum intensity projection (MIP), and shaded surface display (SSD). Nippon Hoshasen Gijutsu Gakkai Zasshi. 2002;58:297-300. |
| 14. | Piotin M, Gailloud P, Bidaut L, Mandai S, Muster M, Moret J, Rüfenacht DA. CT angiography, MR angiography and rotational digital subtraction angiography for volumetric assessment of intracranial aneurysms. An experimental study. Neuroradiology. 2003;45:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Nakayama Y, Imuta M, Funama Y, Kadota M, Utsunomiya D, Shiraishi S, Hayashida Y, Yamashita Y. CT portography by multidetector helical CT: comparison of three rendering models. Radiat Med. 2002;20:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Kim HC, Kim TK, Sung KB, Yoon HK, Kim PN, Ha HK, Kim AY, Kim HJ, Lee MG. CT during hepatic arteriography and portography: an illustrative review. Radiographics. 2002;22:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Calculli L, Casadei R, Amore B, Albini Riccioli L, Minni F, Caputo M, Marrano D, Gavelli G. The usefulness of spiral Computed Tomography and colour-Doppler ultrasonography to predict portal-mesenteric trunk involvement in pancreatic cancer. Radiol Med. 2002;104:307-315. |
| 18. | Choi BI, Lee KH, Han JK, Lee JM. Hepatic arterioportal shunts: dynamic CT and MR features. Korean J Radiol. 2002;3:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Lawler LP, Fishman EK. Extrahepatic arterioportal venous fistula: multidetector CT and volume-rendered angiographic imaging. Abdom Imaging. 2001;26:616-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Häussinger D, Kircheis G. [Hepatic encephalopathy]. Praxis (Bern 1994). 2002;91:957-963. [PubMed] |
| 21. | Federico P, Zochodne DW. Reversible parkinsonism and hyperammonemia associated with portal vein thrombosis. Acta Neurol Scand. 2001;103:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Olson MM, Ilada PB, Apelgren KN. Portal vein thrombosis. Surg Endosc. 2003;17:1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 426] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 24. | Del Piccolo F, Sacerdoti D, Amodio P, Bombonato G, Bolognesi M, Mapelli D, Gatta A. Central nervous system alterations in liver cirrhosis: the role of portal-systemic shunt and portal hypoperfusion. Metab Brain Dis. 2003;18:51-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Luo JJ, Yan ZP, Zhou KR, Qian S. Direct intrahepatic portacaval shunt: an experimental study. World J Gastroenterol. 2003;9:324-328. [PubMed] |
| 27. | Tsai HM, Lin XZ, Chang YC, Lin PW, Hsieh CC. Hepatofugal flow on computed tomography of arterial portography: its correlation with esophageal varices bleeding. Hepatogastroenterology. 2000;47:1615-1618. [PubMed] |
| 28. | Gupta D, Chawla YK, Dhiman RK, Suri S, Dilawari JB. Clinical significance of patent paraumbilical vein in patients with liver cirrhosis. Dig Dis Sci. 2000;45:1861-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Lin J, Zhou KR, Chen ZW, Wang JH, Wu ZQ, Fan J. Three-dimensional contrast-enhanced MR angiography in diagnosis of portal vein involvement by hepatic tumors. World J Gastroenterol. 2003;9:1114-1118. [PubMed] |
| 30. | Otake M, Kobayashi Y, Hashimoto D, Igarashi T, Takahashi M, Kumaoka H, Takagi M, Kawamura K, Koide S, Sasada Y. An inferior mesenteric-caval shunt via the internal iliac vein with portosystemic encephalopathy. Intern Med. 2001;40:887-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Gulati MS, Paul SB, Arora NK, Berry M. Evaluation of extrahepatic portal hypertension and surgical portal systemic shunts by intravenous CT portography. Clin Imaging. 1999;23:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Vignaux O, Gouya H, Augui J, Oudjit A, Coste J, Dousset B, Chaussade S, Legmann P. Hepatofugal portal flow in advanced liver cirrhosis with spontaneous portosystemic shunts: effects on parenchymal hepatic enhancement at dual-phase helical CT. Abdom Imaging. 2002;27:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Haghighat N, McCandless DW, Geraminegad P. The effect of ammonium chloride on metabolism of primary neurons and neuroblastoma cells in vitro. Metab Brain Dis. 2000;15:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Akhtar AJ, Alamy ME, Yoshikawa TT. Extrahepatic conditions and hepatic encephalopathy in elderly patients. Am J Med Sci. 2002;324:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |