Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1659
Revised: February 8, 2004
Accepted: February 18, 2004
Published online: June 1, 2004
AIM: To evaluate the feasibility and safety of the intraarterial chemotherapy of the liver cancer by an interventional method, catheter-port system.
METHODS: Thirty-two catheter-port systems were implanted percutaneously via the femoral artery or subclavian artery. Chemotherapies were performed 0-5 d after the implantation of the catheter-port systems. The mean interval between two sequent chemotherapies was 4 wk. The occurrence of side effects of the implantation was examined clinically.
RESULTS: Implantation of the catheter-port was successful in all patients. Mean patency period was 210 d. One occlusion (3.1%) of the catheter was observed. Displacement of the catheter was observed in one case (3.1%). One patient rated a hematoma in the chest wall as important. Mild hematoma was reported in 8 cases (25%). In 3 of 32 cases (9.4%), mild pain was reported initially, and dysesthesia was reported in seven (21.9%). No patient rated overall discomfort as mild, severe, or important.
CONCLUSION: Percutaneous placement is feasible and safe for liver regional continuous chemotherapy. Compared with surgical placement, the overall complication rate is comparable or less.
- Citation: Zhu AL, Liu LX, Piao DX, Lin YX, Zhao JP, Jiang HC. Liver regional continuous chemotherapy: Use of femoral or subclavian artery for percutaneous implantation of catheter-port systems. World J Gastroenterol 2004; 10(11): 1659-1662
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1659.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1659
Systemic chemotherapy in cases of liver cancer and liver metastases of colorectal cancer has nearly been abandoned due to the high non-response rates. Regional continuously intraarterial chemotherapy has demonstrated better response rates than systemic chemotherapy[1-4]. Percutaneously implantable catheter-port systems have been developed for long-term use to facilitate the long-term administration of chemotherapeutic agents. These systems allow easy and repetitive puncture in infusion therapy without doing much harm to the vessels, and their use is comfortable for the patient. So far, the implantation of permanent intraarterial catheter systems in the gastroduodenal artery[1,5-9] or via the subclavian, axillary, or brachial arteries into the common hepatic artery[10-12] has been performed surgically with considerable complication rates. Moreover, the repair and replacement of malfunctioning port systems previously required surgery[9,13,15,16]. Thus far, percutaneous implantation of catheter-port systems for intraarterial use in various target organs, particularly in regional chemotherapy of the liver, has been successfully performed by radiologists[14,17-20].
From December 1999 to July 2003, 32 percutaneously implantable catheter-port systems (BBRAUN, Germany) were placed in 32 patients (23 men and 9 women; age range, 26-65 years; mean age, 56 years) with primary malignancies of the liver (26 cases) and metastasizes (6 cases). Three patients had Cholangioma within the 25 primary malignancies. In all patients, the catheter-port system implanted percutaneously with radiologic guidance was the first method used to administer intraarterial chemotherapy. One patient had been performed intervention for 2 times because the catheter-port systems failed to be implanted through the femoral arteries in the first performance of intervention. The catheter-ports were implanted percutaneously through subclavian arteries in the second time. We obtained informed consent from each patient prior to the procedure.
The standard catheter-port device consisted of a polysulphone port reservoir with a silicone septum at the puncture site and a lateral stem to slip the silicone catheter over. The connection between the silicone catheter and the polyurethane catheter was reinforced with a small plastic cannula. The port reservoir, polyurethane catheter, cannula, and suture material were commercially available as part of the standard catheter-port system.
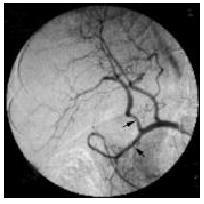
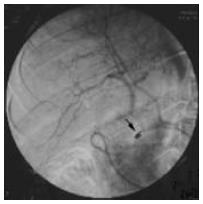
The angiographic catheter was advanced into the respective target vessel such as the common or proper hepatic artery with fluoroscopic guidance after the common femoral or subclavian artery was punctured with use of the Seldinger technique, and visceral arteriography was performed to assess variant arterial supply. The final position of the catheter tip, and thereby the region of perfusion, was chosen according to the anatomy of each patient and the location of the lesion at digital subtraction angiography. The catheter tip was placed into the right hepatic artery (n = 9), the proper hepatic artery (n = 21), and the common hepatic artery (n = 1). The latter position was used in one patient with variant common hepatic artery without the proper hepatic artery (Figure 1). In this patient, the gastroduodenal artery was occluded because of the variant arterial supplies (Figure 2). The correct position of the catheter was verified with digital subtraction angiography.
The catheter-port systems were implanted percutaneously in inpatients. The antibiotic agent was given 20 min before operation and no conscious sedation treatment was administered.
To insert the catheter-port system, an incision approximately 3 cm long was made in the skin distally to the right groin at the anterior surface of the thigh or at the anterior surface of the left or right chest wall, starting from 3 cm distally to the cutaneous puncture site and leading downward along the femoral triangle or leading parallel to the dermatoglyph. A subcutaneous pocket was then formed wherein the port reservoir was to be placed. The end of the catheter was cut off distally to the puncture site, and then was connected tightly to the port reservoir.
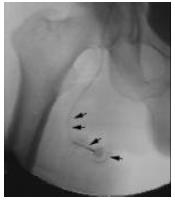
Tunneling was made from the puncture site to the incision and the port reservoir was not fixed to the subcutaneous tissue with an extra suture (Figure 3). Final port angiography helped verify the integrity of the system and correct position of the catheter. When necessary, malposition or dysfunction was corrected before the subcutaneous pocket was closed. A compression bandage was applied in each patient for 24 h.
The patients were mobilized 24 h after the intervention. There were no restrictions on patient activity 24 h after the device was in place. Bed rest was not necessary. By choice, most patients stayed in hospital until the onset of chemotherapy, which was 0-5 d after implantation (mean, 2 d) because of the healing of the incisions or the amelioration of liver function. We regularly flushed the catheter system with 10 mL of heparin sodium (25 eIU/mL heparin) at the end of each chemotherapeutic cycle, and before final withdrawal of the port needle, not between two chemotherapeutic cycles. Patients did not receive any anticoagulation therapy systemically or via the catheter system. At the end of the study, no patients were lost to follow-up. Correct functioning of each catheter-port system was verified with digital subtraction angiography prior to each chemotherapeutic treatment cycle. At each angiographic study, patients were examined clinically for the occurrence of negative side effects, such as peripheral embolization, occlusion. They were also asked to complete a questionnaire regarding their satisfaction with the percutaneously implanted catheter-port system and the presence of local complications, such as hematoma, infection, pain, restriction of motion, dysesthesia, and long-term discomfort. The patients rated these complications as absent, mild, or important. Mild hematoma was defined as discoloration of the skin without subcutaneous swelling or induration for a maximum of 1 wk. Important hematoma was defined as subcutaneous induration with palpable liquid collection around the device of more than 2 cm in diameter.
Implantation of the catheter-port was successful in all patients. Only 2 patients were performed for 2 times because of the failures of implantation through femoral arteries in the first operation. We changed to the subclavian artery and the implantation was successful in the second time. All implantation procedures were performed in the interventional radiology suite with a mean procedure time of 51 min (range, 30-145 min). No peri-interventional complications were noticed. The mean follow-up of the systems was 210 d (range, 36-680 d).
Complications occurred in 2 (6.25%) of 32 cases. One occlusion (1 of 32 cases, 3%) of the catheter was observed on d 36. The occlusion was a result of misuse of heparin solution after the chemotherapy with a subsequent reflux of blood into the catheter. Because lytic therapy with 10000 IU of urokinase in 2 mL of water solution was not successful in this patient, the catheter-port system was disused.
Displacement of the catheter was observed in one case (1 of 32 cases, 3%) at the third angiographic follow-up study. The catheter tip was dislocated into the abdominal artery and could be repositioned into the common hepatic artery by using an interventional maneuver. Since the patient did not want to continue chemotherapy, the port system was entirely disused, but was not removed by operation.
The overall disused rate was 6% (2 of 32 cases). All disused catheter-ports were not removed.
After implantation of a catheter-port system, one patient rated a hematoma in the chest wall as important, but this could not be verified at any clinical follow-up examination, and it did not require surgical intervention. Immediately after implantation, mild hematoma was reported in 8 of 32 cases (25%), but no hemorrhage at the implantation or puncture site was found at the first follow-up examination in any of these cases. In 3 of 32 cases (9%), mild pain was reported initially, and dysesthesia was reported in 7 (22%). No patient rated overall discomfort as mild, severe, or important.
Till July 2003, 145 port angiographic studies were performed as follow-up examinations at a mean interval of 4 wk between each study. The patients were examined clinically for the occurrence of side effects at the time of each angiographic examination. No patients showed signs of peripheral arterial embolization, occlusion, or embolic effects. At all follow-up examinations, we did not find infection, leakage, kinking, or disconnection of any catheter-port system. According to the patient questionnaires, all patients were entirely satisfied with the system. No patient reported restriction of motion or discomfort owing to the port reservoir in the groin or chest wall.
Continuous chemotherapeutic infusion has proved to be prior to the systemic chemotherapy in liver cancer. Permanent percutaneously implanted catheter-port systems are widely used method for it with the advantage for repeated external arterial or central venous access for regional or systemic chemotherapy and prolonged parenteral nutrition[2,6,16]. The majority of catheter-port devices developed for intraarterial regional chemotherapy have necessitated surgical implantation before the interventional method was applied clinically[7,11,21-23]. The common sites of surgical implantation are gastroduodenal, common or proper hepatic arteries for regional intraarterial chemotherapy of the liver.
It has been reported the implantation of an intraarterial catheter-port with fluoroscopic guidance in the interventional radiology suite without laparotomy[8]. The femoral arteries were used for minimally invasive catheter placement. Radiologic implantation is also associated with various complications, such as catheter dislocation, occlusion, and infection. Dislocation and occlusion of catheter are the severest complications, which lead to the termination of chemotherapy or another traumatic implantation procedure[10,12,16,23].
Thrombotic complications such as catheter occlusion and occlusion of the hepatic artery are also commonly associated with both surgically and radiologically implanted catheter-port systems[1,7,9,10,12,23]. Catheter placement via the brachial artery can also be accompanied by thrombosis or occlusion of the brachial artery[12,13]. In our patient group, one case of catheter occlusion was caused by blood reflux into the 4-F catheter. Catheters with small diameters have higher occlusion rates. Niederhuber et al[20] also found that small-bore catheters had an inferior patency rate and drug infusion might be difficult owing to the higher resistance of small catheters. Some infusion pumps might stop at pressure levels that are too high. Therefore, on the basis of results in our study and the literature, we do not recommend the use of catheter below 4-F for permanent implantation in this context.
With regard to prevent catheter thrombosis, different authors have various ideas. Two methods have been recommended: administration of warfarin sodium and continuous catheter perfusion with heparin[18]. We believe that keeping blood from the lumen of catheter and perfusion with the end of each chemotherapy are the efficient methods. We do not consider systemic or other type of anticoagulation therapy necessary. Lytic therapy with tissue plasminogen activator, urokinase, or streptokinase has been reported to be useful in cases of catheter occlusion, but this method is successful only in a few cases[9,16]. In our study, lytic therapy was not effective. But the permanent existence of the disused catheter-port system has no harm to the patient if there is no septic episode.
The frequency of dislocation appeared to be particularly high when the axillary or brachial artery was used[10,12]. It could be due to the too soft and flexible catheter material and the mobility of the upper limb. In our study, displacement occurred in one of our cases (3.1%), in which the port reservoir was implanted in the chest wall through right subclavian artery, into the abdominal artery. Retrospectively, we believe this displacement into the abdominal artery was probably due to too much tension on the indwelling catheter and the mobility of upper limb. Therefore, optimal catheter configuration and the approach are crucial. We recommend that the right femoral artery should be the best approach.
Infection is another complication in permanently implanted catheter systems that often makes removal of the device necessary[8,19,24,29,30]. Infection rates ranged from 0%[25-28] to 7.6%[11,17,23,24]. Infection and sepsis during chemotherapy can be caused by the use of inappropriate hygienic measures and can be treated successfully with antibiotic therapy. It has been noticed that after an infected catheter-port system was removed and replaced with a new one, however, infection recurred in some patients[14]. In our study patients, antibiotic agents were administered 20 min before operation for the aim of prophylaxis of infection, and infection was not observed. Infection rates after radiologic implantation are lower than those after surgical implantation. Therefore, interventional radiology suites and the antibiotic agent seem to provide sufficient hygienic conditions for this type of intervention.
The relatively low complication rate and pain increase patient acceptance of this procedure. Placement of the catheter-port system on the anterior surface of the thigh below the groin or chest wall seems to be well accepted, even in very active patients. Its superficial placement allows easy palpation and puncture, and provides little risk for dislocation or disconnection of the port needle from the reservoir during chemotherapy. But careful palpations are required in obese patients.
Radiologic implantation of catheter-port systems is a quick and simple procedure that does not require general anesthesia compared with surgical implantation. Patency rates are equal to or higher than those for surgically implanted systems. Radiologic placement is also possible in patients with anatomic vascular variations. In contrast to the surgical method, catheter-port systems placed radiologically cause less morbidity in the case of dysfunction, because the systems can be removed or repositioned more easily. Complicated surgical revisions or corrections requiring laparotomy can be avoided as proposed by Doughty et al[31]. Radiologic placement does not allow performance of preventive cholecystectomy to avoid cholecystitis, but this does not seem to be a crucial problem.
Our results indicate that percutaneous implantation of a catheter-port system via the femoral artery or subclavian artery is easy to perform, simplifies intraarterial chemotherapy of the liver with equal patency rates, and has fewer complications as compared with surgical placement, and is well accepted by patients.
Edited by Kumar M Proofread by Xu FM
| 1. | de Takats PG, Kerr DJ, Poole CJ, Warren HW, McArdle CS. Hepatic arterial chemotherapy for metastatic colorectal carcinoma. Br J Cancer. 1994;69:372-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Sterchi JM. Hepatic artery infusion for metastatic neoplastic disease. Surg Gynecol Obstet. 1985;160:477-489. [PubMed] |
| 3. | Hohn DC, Stagg RJ, Friedman MA, Hannigan JF, Rayner A, Ignoffo RJ, Acord P, Lewis BJ. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646-1654. [PubMed] |
| 4. | Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 387] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Kemeny N, Seiter K, Conti JA, Cohen A, Bertino JR, Sigurdson ER, Botet J, Chapman D, Mazumdar M, Budd AJ. Hepatic arterial floxuridine and leucovorin for unresectable liver metastases from colorectal carcinoma. New dose schedules and survival update. Cancer. 1994;73:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Link KH, Sunelaitis E, Kornmann M, Schatz M, Gansauge F, Leder G, Formentini A, Staib L, Pillasch J, Beger HG. Regional chemotherapy of nonresectable colorectal liver metastases with mitoxantrone, 5-fluorouracil, folinic acid, and mitomycin C may prolong survival. Cancer. 2001;92:2746-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Strecker EP, Ostheim-Dzerowycz W, Boos IB. Intraarterial infusion therapy via a subcutaneous port for limb-threatening ischemia: a pilot study. Cardiovasc Intervent Radiol. 1998;21:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Oberfield RA, McCaffrey JA, Polio J, Clouse ME, Hamilton T. Prolonged and continuous percutaneous intra-arterial hepatic infusion chemotherapy in advanced metastatic liver adenocarcinoma from colorectal primary. Cancer. 1979;44:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Ekberg H, Tranberg KG, Lundstedt C, Hanff G, Ranstam J, Jeppsson B, Bengmark S. Determinants of survival after intraarterial infusion of 5-fluorouracil for liver metastases from colorectal cancer: a multivariate analysis. J Surg Oncol. 1986;31:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Okuyama K, Tohnosu N, Koide Y, Awano T, Matsubara H, Sano T, Nakaichi H, Funami Y, Matsushita K, Kikuchi T. [Complications and their management in intraarterial infusion chemotherapy]. Gan To Kagaku Ryoho. 1992;19:1007-1013. [PubMed] |
| 11. | Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994;344:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 366] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Laffer U, Dürig M, Bloch HR, Zuber M, Stoll HR. [Implantable catheter systems. Experience with 205 patients]. Dtsch Med Wochenschr. 1989;114:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Germer CT, Boese-Landgraf J, Albrecht D, Wagner A, Wolf KJ, Buhr HJ. [The fully implantable minimally invasive hepatic artery catheter for locoregional chemotherapy of nonresectable liver metastases in defective conventional implanted therapy catheters]. Chirurg. 1996;67:458-462. [PubMed] |
| 14. | Kuroiwa T, Honda H, Yoshimitsu K, Irie H, Aibe H, Shinozaki K, Nishie A, Nakayama T, Masuda K. [A safe and simple method of percutaneous transfemoral implantation of a port-catheter access system for hepatic artery chemotherapy infusion]. Gan To Kagaku Ryoho. 2001;28:1573-1577. [PubMed] |
| 15. | Pullyblank AM, Carey PD, Pearce SZ, Tanner AG, Guillou PJ, Monson JR. Comparison between peripherally implanted ports and externally sited catheters for long-term venous access. Ann R Coll Surg Engl. 1994;76:33-38. [PubMed] |
| 16. | Harvey WH, Pick TE, Reed K, Solenberger RI. A prospective evaluation of the Port-A-Cath implantable venous access system in chronically ill adults and children. Surg Gynecol Obstet. 1989;169:495-500. [PubMed] |
| 17. | Civalleri D, Cafiero F, Cosimelli M, Craus W, Doci R, Repetto M, Simoni G. Regional arterial chemotherapy of liver tumours. I. Performance comparison between a totally implantable pump and a conventional access system. Eur J Surg Oncol. 1986;12:277-282. [PubMed] |
| 18. | Huk I, Entscheff P, Prager M, Schulz F, Polterauer P, Funovics J. Patency rate of implantable devices during long-term intraarterial chemotherapy. Angiology. 1990;41:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Ross MN, Haase GM, Poole MA, Burrington JD, Odom LF. Comparison of totally implanted reservoirs with external catheters as venous access devices in pediatric oncologic patients. Surg Gynecol Obstet. 1988;167:141-144. [PubMed] |
| 20. | Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706-712. [PubMed] |
| 21. | Wopfner F. [Treatment of inoperable liver metastases: preliminary results with intrahepatic chemotherapy via a new type of indwelling catheter (author's transl)]. Dtsch Med Wochenschr. 1981;106:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Balch CM, Urist MM, McGregor ML. Continuous regional chemotherapy for metastatic colorectal cancer using a totally implantable infusion pump. A feasibility study in 50 patients. Am J Surg. 1983;145:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Buchwald H, Grage TB, Vassilopoulos PP, Rohde TD, Varco RL, Blackshear PJ. Intraarterial infusion chemotherapy for hepatic carcinoma using a totally implantable infusion pump. Cancer. 1980;45:866-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Dresing K, Lottner C, Stock W. [Port-catheter perforation into the duodenum and other early complications after port implantation before intra-arterial infusion therapy of the liver with chemotherapeutic drugs]. Med Klin (Munich). 1991;86:245-250. [PubMed] |
| 25. | al-Hathal M, Malmfors G, Garwicz S, Békássy AN. Port-A-Cath in children during long-term chemotherapy: complications and outcome. Pediatr Hematol Oncol. 1989;6:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Hohn DC, Rayner AA, Economou JS, Ignoffo RJ, Lewis BJ, Stagg RJ. Toxicities and complications of implanted pump hepatic arterial and intravenous floxuridine infusion. Cancer. 1986;57:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Strecker EP, Boos IB, Ostheim-Dzerowycz W, Heber R, Vetter SC. Percutaneously implantable catheter-port system: preliminary technical results. Radiology. 1997;202:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Fuchs R, Leimer L, Koch G, Westerhausen M. [Clinical experience with bacterial contamination of Port-A-Cath systems in tumor patients]. Dtsch Med Wochenschr. 1987;112:1615-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Zanon C, Grosso M, Zanon E, Veltri A, Alabiso O, Bazzan M, Chiappino I, Mussa A. Transaxillary access to perform hepatic artery infusion (HAI) for secondary or primitive hepatic tumors. Minerva Chir. 1996;51:755-758. [PubMed] |
| 30. | Bern MM, Lokich JJ, Wallach SR, Bothe A, Benotti PN, Arkin CF, Greco FA, Huberman M, Moore C. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 585] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | Doughty JC, Keogh G, McArdle CS. Methods of replacing blocked hepatic artery catheters. Br J Surg. 1997;84:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |