Published online Jun 1, 2004. doi: 10.3748/wjg.v10.i11.1594
Revised: February 4, 2004
Accepted: February 11, 2004
Published online: June 1, 2004
AIM: To investigate the role of Peyer’s patch lymphocytes in the regulation of enteric epithelial barrier and ion transport function in homeostasis and host defense.
METHODS: Mouse Peyer’s patch lymphocytes were co-cultured with human intestinal epithelial cell line Caco-2 either in the mixed or separated (isolated but permeable compartments) culture configuration. Barrier and transport functions of the Caco-2 epithelial monolayers were measured with short-circuit current (Isc) technique. Release of cytokines was measured by enzyme-linked immunosorbent assay (ELISA) and cytokine mRNA expression was analyzed by semi-quantitative RT-PCR. Barrier and iontransport functions of both culture conditions following exposure to Shigella lipopolysaccharide (LPS) were also examined.
RESULTS: The transepithelial resistance (TER) of the epithelial monolayers co-cultured with Peyer’s patch lymphocytes was maintained whereas that of the Caco-2 monolayers alone significantly decreased after eight days in culture. The forskolin-induced anion secretion, in either absence or presence of LPS, was significantly suppressed in the both co-cultures as compared with the Caco-2 cells alone. Furthermore, only the mixed co-culture condition induced the expression and release of mIL-6 from Peyer’s patch lymphocytes, which could be further enhanced by LPS. However, both co-culture conditions suppressed expression and release of epithelial hIL-8 under the unstimulated conditions, while the treatment with LPS stimulated their hIL-8 expression and release.
CONCLUSION: Peyer’s patch lymphocytes may modulate intestinal epithelial barrier and ion transport function in homeostasis and host defense via cell-cell contact and cytokine signaling.
- Citation: Chen J, Tsang LL, Ho LS, Rowlands DK, Gao JY, Ng CP, Chung YW, Chan HC. Modulation of human enteric epithelial barrier and ion transport function by Peyer’s patch lymphocytes. World J Gastroenterol 2004; 10(11): 1594-1599
- URL: https://www.wjgnet.com/1007-9327/full/v10/i11/1594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i11.1594
The mucosal surface of the gastrointestinal tract is lined by a single layer of epithelial cells jointed together at their apical poles by tight junctions, forming a barrier that separates the luminal contents from the effector immune cells underneath. It has become increasingly clear that the intense immunological activities occurring at the enteric mucosal surface involve interactions between epithelial and immune cells[1,2]. Accumulating evidence suggests that epithelial cells can produce cytokines and chemokines that attract and activate immune cells with potentially important effects on the immediate and long-term host defense functions. Effective immune surveillance of the mucosal surface requires transport of intact macromolecules and micro-organisms across the epithelial barrier to the cells of mucosal immune system. Groups of organized mucosal lymphoid follicles, named Peyer’s patches, lined along the gastrointestinal tract in a specialized overlying epithelium, are the sites for transporting, processing and presentating foreign antigens. In Peyer’s patches, each follicle is separated from the overlying epithelium by a subepithelial “dome” region that is rich in lymphocytes and dendritic cells. It is apparent that the immune cells in the dome region interact intimately with the overlying epithelium, giving rise to mucosal immune response without the needs of systemic involvement[3]. Although a mucosal lympho-epithelial internet has been proposed to mediate the interactions between epithelial and immune cells, details of the interactions are far from understood. The close contact between Peyer’s patch lymphocytes and intestinal epithelial cells suggests that these lymphocytes may play a role in the modulation of epithelial barrier/transport functions in homeostasis as well as host defense. However, no evidence has been provided so far concerning the role of Peyer’s patch lymphocytes in intestinal epithelial physiology.
In addition to their major defensive role as a passive barrier, the intestinal epithelial cells also play an active role in physiology and pathophysiology of the gut. While the absorptive properties of the epithelium are known to be of vital importance for the nutrition of the body, as well as the maintenance electrolytes and fluid balance, the secretory activities of the epithelium are also important for protective purpose. Luminally directed transport of Cl- and HCO3- is the driving force for fluid secretion that flushes away noxious substances from the intestinal mucosal surface. Secretory diarrhea is often the consequence for increased water and electrolyte secretion upon invasion by microorganisms. Escherichia, Salmonella and Shigella are the most important bacterial causes of diarrhea worldwide[4]. The pathogenesis of bacteria-induced diarrhea with regard to the involvement of Peyer’s patch lymphocytes remains largely unknown although the barrier function and ion secretory responses of the intestinal epithelium have been shown to be affected by both proinflammatory and anti-inflammatory cytokines[5-7].
The co-culture of characterized epithelial cells with defined immune cell populations and subsequent analysis of epithelial physiology have contributed significantly to our appreciation of the immune regulation of epithelial function[8,9]. Kerneis et al[10] have successfully induced functional M cells by co-culturing Caco-2 cells, a human intestinal surface epithelial cell line, with murine Peyer’s patch lymphocytes. This phenomenon indicates the profound influence of Peyer’s patch lymphocytes on intestinal epithelial phenotypes[11]. Thus, to investigate the role of Peyer’s patch lymphocytes in intestinal epithelial barrier/transport function during infection, we adopted this co-culture system to build an infection model with Shigella LPS, one of the major virulence factors of Gram-negative bacteria that is responsible for eliciting a wide array of immune responses[11,12]. The present study includes different co-culture configurations: One consisting of upper and lower compartments with a permeable filter separating the epithelial layer and lymphocytes, and the other mixing both epithelial cells and lymphocytes in the same compartment. Using these co-culture configurations, epithelial-immune interactions through cell-cell contact as well as cytokine signaling could be examined independently.
BALB/c mice (SPF, female, 6-8 wk old) were obtained from the Animal House of Chinese University of Hong Kong. The lymphoid follicles of the Peyer’s patch were carefully excised from the intestinal serosal side and placed in 10 mL phosphate buffered saline (PBS, pH7.4, GIBCO 10010-31) supplemented with 20 mL/L FBS and 2% penicillin-streptomycin. The collected patches were triturated by pipetting up and down a few times and smashing through a metallic grid (mesh: 100). Individual lymphocytes were released in the liquid below the metallic grid. The lymphocytes were washed with PBS, the distribution of Peyer’s patch T and B cell populations was consistent with previous data when they were checked by flow cytometry[10], then diluted to the expected concentration.
Human colonic cell line Caco-2, which is a villus cell-like colonic cell line[13], was purchased from American Type Culture Collection (Rockville, MD). The cells were grown in Dulbecco Modified Eagle’s minimal essential medium (DMEM, high glucose, GIBC-BRL) with 100 mL/L fetal bovine serum (FBS, GIBCO 16000-044), 2 mmol/L L-glutamine (GIBCO-BRL), 100 mmol/L non-essential amino acid (GIBCO-BRL), 200 units/mL penicillin and 200 mg/mL streptomycin (GIBCO-BRL) at 37 °C in a 50 mL/L CO2 atmosphere. Caco-2 cells were seeded at a density of 3 × 105 cells on a floating permeable support, which was made of a membrane filter (Millipore, 0.45 mm pore size) with a silicone rubber ring attached on top of it for confining the cells (0.45 cm2 growth area).
Three types of culture configurations were established in this study: (1) Caco-2 culture alone: Caco-2 cells (3 × 105 cells) were grown as a homogenous polarized monolayer according to the epithelial cell culture method mentioned above and served as the control. (2) Separated co-culture: Epithelial cells (3 × 105 cells) were plated on a permeable support (growth area of 0.45 cm2 as described), which floated above 2 mL culture medium containing Peyer’s patch lymphocytes (5 × 106 cells) in a Petri dish (3.5-cm diameter). (3) Mixed co-culture: Caco-2 cells (3 × 105 cells) were directly mixed with Peyer’s patch lymphocytes (1 × 106 cells), and then were seeded on the same permeable support described above and grown at 37 °C in a 50 mL/L CO2 atmosphere.
When the Caco-2 cells grown as polarized monolayers on the membrane filter reached confluence on the 4th d, Shigella F2a-12 LPS (5 mg/mL, obtained from the Immunology Department of Institute of Microbiology & Epidemiology, Academy of Military Medical Sciences) was added to the apical side of the epithelial monolayers and treated for 8, 24 and 48 h at 37 °C in a 50 mL/L CO2 atmosphere.
The Isc measurement has been described previously[14]. In short, the confluent monolayers were clamped vertically between the two halves of an Ussing chamber. Monolayers were short circuited (transepithelial potential difference clamped at zero) using a voltage-clamp amplifier (DVC-1000; World Precision Instruments Inc., New Haven, CT, USA). The resulting Isc was displayed on-line on a pen recorder (Kipp and Zonen, Delft, The Netherlands). Transepithelial electrical resistance (TER) was determined based on Ohm’s law by clamping the tissue intermittently at a value of 0.2 mV. For most measurements, the monolayers were bathed in normal Krebs-Henseleit solutions (NaCl, 117 mmol/L ; KCl, 4.7 mmol/L; MgCl2, 1.2 mmol/L; NaHCO3, 24.8 mmol/L; KH2PO4, 1.2 mmol/L; CaCl2, 2.56 mmol/L; Glucose, 11.1 mmol/L) with 950 mL/L O2 and 50 mL/L CO2.
Caco-2 monolayers grown on the filters (Millipore, 0.22 mm pore size, cell growth area of 7.065 cm2) were harvested for RT-PCR after 8-h and 24-h Shigella F2a-12 LPS pre-treatment. The specific primers for hIL-8[15] were 5’ tct ctt ggc agc ctt cct 3’ (sense) and 5’ gaa gtt tca ctg gca tct tca c 3’ (antisense), corresponding to the nucleotides 98-427 with the expected cDNA size of 390 bp (Tm 58 °C, 30 cycle) ; mIL-6[16] were 5’ ctg caa gag act tcc atc cag 3’ (sense) and 5’ tcc agt ttg gta gca tcc atc 3’ (antisense), corresponding to the nucleotides 45-340 with the expected cDNA size of 296 bp (Tm 55 °C, 35 cycle). Their expression was compared to the house-keeping gene GAPDH (forward: 5’ tcc cat cac cat ctt cca g 3’ and reverse: 5’ tcc acc act gac acg ttg 3’). RT-PCR was performed using the PTC-200 Peltier Thermal Cycler (MJ Research Company) and software for analysis data is GraphPad Prism 3.02.
After confluent Caco-2 monolayers on the Millipore filter was challenged by Shigella F2a-12 LPS pretreatment for 8 and 24 h, the culture medium was collected and kept at -20 °C until evaluation hIL-8 and mIL-6 bioassay using ELISA kits (BIOSOURCE Company) according to manufacturer’s instructions.
All data were expressed as mean ± SE. The number of experiments represents independent measurements on separate monolayers. Comparisons between groups of data were made by one-way ANOVA. A “P” value of less than 0.05 was considered statistically significant.
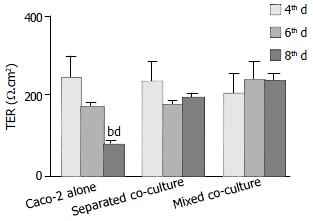
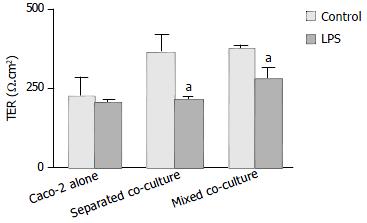
Polarized monolayers of Caco-2 were tested for transepithelial resistance (TER) at various times (the 4th, 6th and 8th d after co-culture) (Figure 1). A significant decrease in TER was observed in Caco-2 alone after eight days in culture, whereas high TER could still be maintained for either mixed or separated co-cultures for the same period of time (Figure 1). However, after treatment with Shigella LPS (5 mg/mL) for 48 h, TER measured on the 6th d of co-culture, decreased significantly in both co-culture groups but not in Caco-2 alone (Figure 2).
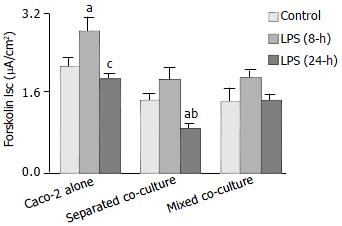
The Isc responses of the co-cultures and Caco-2 alone to the challenge of an adenylate cyclase activator, forskolin, were characterized. As shown in Figure 3, significantly lower Isc response was observed in both co-cultures than that of the Caco-2 alone control in the absence of LPS. Increased responses in all groups were observed after 8-h LPS treatment. Significantly more upregulated Isc response was observed in Caco-2 alone group (Figure 3). After 24 h of LPS treatment, the Isc responses of the separated co-culture and Caco-2 alone groups decreased significantly as compared with that of 8-h LPS treatment but not the mixed co-culture (Figure 3). No significant differences in the basal Isc among the groups were observed, nor did it change significantly upon treatment with LPS (data not shown).
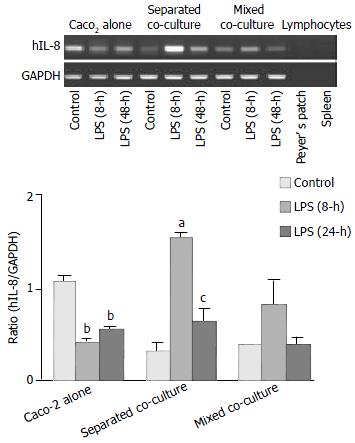
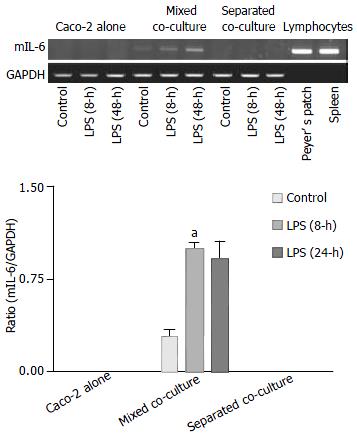
To examine the mRNA expression of hIL-8, primers specific for hIL-8 were used in RT-PCR experiments, and the RNA from mouse Peyer’s patch lymphocytes was used as negative control to detect any potential. The results are shown in Figure 4. The highest level of hIL-8 expression was observed in the Caco-2 alone as compared to the other two co-cultures when they were cultured in the absence of LPS. However, after treatment with LPS for 8 h, lower hIL-8 expression was observed in the Caco-2 culture alone while increased hIL-8 expression was evident in both co-cultures. The increase in the hIL-8 expression was particularly more enhanced in the separated co-culture. After 24 h, the expression of hIL-8 in the co-cultures returned closer to their basal levels but the expression in the Caco-2 alone remained low as compared with its own control. The expression of hIL-8 was not detected in mouse Peyer’s patch lymphocytes confirming that the above expression profile of hIL-8 was associated with human intestinal epithelial cells.
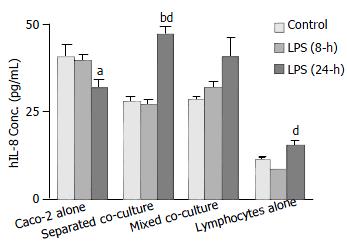
ELISA using a human kit was conducted to confirm the release of hIL-8 from different cultures with mouse Peyer’s patch lymphocytes as a control for cross-reactivity. The results showed that the levels of hIL-8 released from the Caco-2 alone culture under the unstimulated condition and 8-h LPS treatment were significantly greater than that from both co-cultures (Figure 5). However, following the 24-h LPS treatment, levels of hIL-8 in both co-culture groups increased considerably while a decrease was observed in Caco-2 alone (Figure 5). A detectable amount of hIL-8 was also observed from mouse Peyer’s patch lymphocytes (1 × 106 cells), indicating the presence of condition cross-reactivity of human IL-8 antibody to mouse IL-8 (Figure 5).
Using primers specific for mIL-6, the expected RT-PCR product of mIL-6 was detected in the mixed co-culture and mouse lymphocytes but not in the Caco-2 culture alone or separated co-culture, where only the epithelial cells in the upper compartment were subjected to RT-PCR, thus excluding detection of IL-6 from the Peyer’s patch lymphocytes in the medium. The expression of mIL-6 in the mixed co-culture further increased after 8 and 24 h of treatment with LPS (Figure 6).
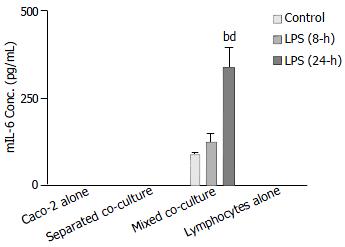
The release of mIL-6 from different cultures, as well as mouse Peyer’s patch lymphocytes (1 × 106 cells), was measured using a mouse ELISA kit. The results showed that no groups except the mixed co-culture had detectable mIL-6 release either in the absence or presence of LPS treatment (Figure 7). The level of mIL-6 measured in the mixed co-culture group significantly increased after 24-h LPS treatment (Figure 7).
The present study has demonstrated for the first time the role of Peyer’s patch lymphocytes in intestinal epithelial physiology in homeostasis as well as host defense. Both epithelial barrier function and anion secretory activities, under normal or infected conditions, were affected by the presence of Peyer’s patch lymphocytes to various extent depending on the co-culture configurations. TER has been used as an index of epithelial barrier integrity, which is sometimes compromised in certain infections, possibly as a direct result of modulation by immune cells[17]. Previous studies in co-cultures of epithelial cells and immune cells, including lamina propria mononuclear cells (LPMC)[18] and intraepithelial lymphocytes[19], invariably resulted in disruption of epithelial barrier function as indicated by a decrease in TER. However, the present study did not observe significant reduction in the TER in the Caco-2 co-cultures with Peyer’s patch lymphocytes, either in the separated or mixed configuration, as compared with the Caco-2 culture alone. On the contrary, the TER in both co-cultures could be maintained up to eight days whereas that in Caco-2 control decreased drastically. These results suggest that unlike other types of immune cells previously studied, Peyer’s patch immune cells, which consist mostly of lymphocyte (99%)[10], play a distinct homeostatic regulatory role in maintaining the barrier function of the epithelial monolayers. However, TER of both co-cultures decreased substantially after treatment with Shigella LPS for 48 h, indicating that Shigella LPS may have direct effect on epithelial barrier function without the modulation of Peyer’s patch lymphocytes. This notion is also supported by the observed mRNA expression of tight junction-associated protein ZO-1 in both co-cultures with Peyer’s patch lymphocytes (data not shown). It has been shown that LPS accounts for a large part of the transepithelial signaling caused by apical Shigella bacteria that induces adherence and transmigration of basal polymorphonuclear leukocytes (PMNs), which disrupt the monolayer permeability and facilitate bacterial invasion[20].
Similar to the barrier function, the epithelial secretory activities, in both homeostasis and infection, were also affected by the presence of Peyer’s patch lymphocytes, depending on the configuration of the co-cultures. In normal conditions (or in the absence of LPS), reduced forskolin-induced Isc was observed when Peyer’s patch lymphocytes were co-cultured with Caco-2 and the Isc response in the Caco-2 alone group was significantly increased after LPS challenge up to eight hours. These results suggest that the forskolin-induced Isc response could be suppressed by Peyer’s patch lymphocytes. However, down-regulation of forskolin-induced anion secretion could be seen in all groups 24 h after LPS challenge, suggesting that cytokine signaling also plays a role at later stages of infection, perhaps affecting the expression of ion channels. Interestingly, the Isc response of anion secretion in the mixed co-culture showed no significant change in the absence or presence of LPS, suggesting that cell-cell contact is important for modulation of epithelial anion secretion by Peyer’s patch lymphocytes. In contrast to many previous results obtained from in vitro epithelial model cells showing anion secretion stimulated by bacteria[21] or immune and inflammatory modulators produced by inflammatory cells[22], the present results show no change in the forskolin-induced anion secretion in the mixed co-cultures in the absence or presence of LPS. Reduced secretory responsiveness has also been observed in the epithelial cell models that are co-cultured with a number of different immune cells[7]. This is also consistent with the observation that colonic mucosa from patients with chronic inflammatory bowl disease respond poorly to secretagogues[23]. Thus, it appears that the epithelial secretory response in homeostasis and infection is modulated by immune cells including Peyer’s patch lymphocytes. The present results showed that Peyer’s patch lymphocytes suppressed epithelial secretory activity, especially upon LPS challenge, suggesting that the secretory diarrhea caused by bacterial infection may not be solely due to enhanced water and electrolyte secretion. Recent studies have suggested that the inhibition of Na+-K+-ATPase by interferon g that leads to the reduction of Na+ absorption may be a major cause of inflammation-associated diarrhea[24]. The inhibition of Na+-K+-ATPase induced by interferon g may also account for the presently observed Peyer’s patch lymphocyte-suppressed anion secretion since secondary active anion secretion depends on the Na+ gradient generated by the Na+-K+-ATPase. Further studies examining the expression of various ion channels and transporters including Na+-K+-ATPase and CFTR in response to LPS challenge are currently undertaken in our laboratory.
The present results suggest that the modulation of epithelial physiology by Peyer’s patch lymphocytes involves both cell-cell contact and cytokine signaling. The mouse-human hybrid co-cultures in different configurations, mixed and separated, not only allow the distinction of contact-dependent and independent signaling mechanisms but also the identification of the source of cytokines, although homology between human and mouse cytokine may result in cross-reactivity as seen in the ELISA results showing detectable hIL-8 measured from mouse Peyer’s patch lymphocytes using a human kit. Further experiment with RT-PCR using species-specific primers could also be conducted to confirm the source of cytokines. In fact, the ELISA results were closely correlated with the RT-PCR results with a time lag in the response to LPS. This observation is consistent with the fact that the transcriptional changes occur prior to the translational changes[25].
The more prominent effects on forskolin-induced anion secretion observed in the mixed co-culture as compared to that in the separated co-culture indicate the importance of cell-cell contact in addition to cytokine communication. In fact, cell-cell contact appears to be absolutely required for the enhancement of the expression and release of mIL-6 from Peyer’s patch lymphocytes under both normal condition and LPS challenge. This observation is of physiological significance considering the close contact between Peyer’s patch lymphocytes and intestinal epithelial cells in the Peyer’s patches. IL-6 is known to be produced by a number of cell types including antigen-presenting cells and B cells and it is involved in the acute phase response, B cell maturation and macrophage differentiation[26]. IL-6 has also been reported to activate transcription of a variety of molecules including cytokines and receptors[27]. Therefore, cell-cell contact-induced IL-6 release from Peyer’s patch lymphocytes may affect the expression of epithelial ion channels and transporters and lead to modulation of epithelial function. On the other hand, more enhanced expression and release of hIL-8 from Caco-2 cells was observed in the separated co-culture than the mixed co-culture. This is consistent with the properties of IL-8 as a chemokine for attracting immune cells to the site of inflammation since the separation of Peyer’s patch lymphocytes from Caco-2 cells in the separated co-culture presents a need for IL-8 to signal the recruitment of lymphocytes. However, there is no need for production of IL-8 if the lymphocytes are present next to the epithelial cells as in the mixed co-culture. Epithelial hIL-8 production has been implicated in the disruption of the epithelial barrier function by Shigella[28] as well as other entroinvasive bacteria[29]. Bacterial invasion turns the infected epithelial cells to become strongly proinflammatory with subsequent IL-8 production that attracts polymorphonuclear leukocytes (PMNs), ultimately leading to the disruption of the epithelium. This study only investigated the mIL-6 and hIL-8 expression and release from lymphocytes and epithelial cell to illustrate possible interactions through soluble factors between epithelial and immune cells. Other cytokines may also play a role in the regulation of epithelial physiology by Peyer’s patch lymphocytes. The observed expression and release of mIL-6 and hIL-8, constitutively and in response to the LPS challenge, suggest that they may be involved in mediating the modulatory effects of Peyer’s patch lymphocytes on epithelial barrier and ion transport function in homeostasis and host defense. Further studies are required to identify their specific roles as well as the involvement of other immune mediators in the modulation of epithelial physiology.
In summary, being the sensory arm of the intestinal mucosa, Peyer’s patches are not only involved in transporting, processing and presentation of antigens/pathogens that are essential to eliciting mucosal immune response, but also play an important role in modulating epithelial physiology. This function is distinct from other types of lymphocytes previously studied. Our results indicated that lymphocytes in Peyer’s patches receive information from mucosal surface epithelial cells, via both cell-cell contact and cytokine signaling, and in return modulate epithelial barrier and ion transport function in both homeostasis and host defense. Since immune modulation determines the epithelial responsiveness to infection, the co-cultures of epithelial cells with Peyer’s patch lymphocytes may provide useful models in studying the initiation process involved in the pathogenesis of inflammation-associated diarrhea.
Edited by Ma JY and Xu FM
| 1. | Berin MC, McKay DM, Perdue MH. Immune-epithelial interactions in host defense. Am J Trop Med Hyg. 1999;60:16-25. [PubMed] |
| 2. | Hamzaoui N, Pringault E. Interaction of microorganisms, epithelium, and lymphoid cells of the mucosa-associated lymphoid tissue. Ann N Y Acad Sci. 1998;859:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 394] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Farthing MJ. Novel targets for the pharmacotherapy of diarrhoea: a view for the millennium. J Gastroenterol Hepatol. 2000;15 Suppl:G38-G45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Beltinger J, McKaig BC, Makh S, Stack WA, Hawkey CJ, Mahida YR. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol. 1999;277:C271-C279. [PubMed] |
| 6. | Lu J, Philpott DJ, Saunders PR, Perdue MH, Yang PC, McKay DM. Epithelial ion transport and barrier abnormalities evoked by superantigen-activated immune cells are inhibited by interleukin-10 but not interleukin-4. J Pharmacol Exp Ther. 1998;287:128-136. [PubMed] |
| 7. | McKay DM, Singh PK. Superantigen activation of immune cells evokes epithelial (T84) transport and barrier abnormalities via IFN-gamma and TNF alpha: inhibition of increased permeability, but not diminished secretory responses by TGF-beta2. J Immunol. 1997;159:2382-2390. [PubMed] |
| 8. | McKay DM, Croitoru K, Perdue MH. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol. 1996;270:C418-C428. [PubMed] |
| 9. | Kanzato H, Manabe M, Shimizu M. An in vitro approach to the evaluation of the cross talk between intestinal epithelium and macrophages. Biosci Biotechnol Biochem. 2001;65:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 465] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Kernéis S, Caliot E, Stubbe H, Bogdanova A, Kraehenbuhl J, Pringault E. Molecular studies of the intestinal mucosal barrier physiopathology using cocultures of epithelial and immune cells: a technical update. Microbes Infect. 2000;2:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Takahashi A, Iida T, Naim R, Naykaya Y, Honda T. Chloride secretion induced by thermostable direct haemolysin of Vibrio parahaemolyticus depends on colonic cell maturation. J Med Microbiol. 2001;50:870-878. [PubMed] |
| 14. | Cuthbert AW, George AM, MacVinish L. Kinin effects on electrogenic ion transport in primary cultures of pig renal papillary collecting tubule cells. Am J Physiol. 1985;249:F439-F447. [PubMed] |
| 15. | Rodríguez BL, Rojas A, Campos J, Ledon T, Valle E, Toledo W, Fando R. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect Immun. 2001;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98:9014-9019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Willemsen LE, Schreurs CC, Kroes H, Spillenaar Bilgen EJ, Van Deventer SJ, Van Tol EA. A coculture model mimicking the intestinal mucosa reveals a regulatory role for myofibroblasts in immune-mediated barrier disruption. Dig Dis Sci. 2002;47:2316-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Taylor CT, Murphy A, Kelleher D, Baird AW. Changes in barrier function of a model intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut. 1997;40:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Sansonetti PJ. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens. The paradigm of Shigella. Folia Microbiol (Praha). 1998;43:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: role of iNOS and COX-2. Gastroenterology. 2002;122:1070-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | O'Loughlin EV, Pang GP, Noltorp R, Koina C, Batey R, Clancy R. Interleukin 2 modulates ion secretion and cell proliferation in cultured human small intestinal enterocytes. Gut. 2001;49:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Sandle GI, Higgs N, Crowe P, Marsh MN, Venkatesan S, Peters TJ. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97-105. [PubMed] |
| 24. | Sugi K, Musch MW, Field M, Chang EB. Inhibition of Na+,K+-ATPase by interferon gamma down-regulates intestinal epithelial transport and barrier function. Gastroenterology. 2001;120:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Pédron T, Thibault C, Sansonetti PJ. The invasive phenotype of Shigella flexneri directs a distinct gene expression pattern in the human intestinal epithelial cell line Caco-2. J Biol Chem. 2003;278:33878-33886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 672] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 27. | Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Sansonetti PJ. Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G319-G323. [PubMed] |
| 29. | Fleckenstein JM, Kopecko DJ. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J Clin Invest. 2001;107:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |