Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.91
Revised: July 20, 2003
Accepted: July 24, 2003
Published online: January 1, 2004
AIM: To better clarify the main target organs of dimethylarsinic acid toxicity and the role of metallothionein (MTs) in modifying dimethylarsinic acid (DMAA) toxicity.
METHODS: MT-I/II null (MT- /-) mice and the corresponding wild-type mice (MT+/+), six in each group, were exposed to DMAA (0-750 mg/kg body weight) by a single oral injection. Twenty four hours later, the lungs, livers and kidneys were collected and undergone pathological analysis, induction of apoptotic cells as determined by TUNEL and MT concentration was detected by radio-immunoassay.
RESULTS: Remarkable pathological lesions were observed at the doses ranging from 350 to 750 mg/kg body weight in the lungs, livers and kidneys and MT+/+ mice exhibited a relatively slight destruction when compared with that in dose matched MT- /- mice. The number of apoptotic cells was increased in a dose dependent manner in the lungs and livers in both types of mice. DMAA produced more necrotic cells rather than apoptotic cells at the highest dose of 750 mg/kg, however, no significant increase was observed in the kidney. Hepatic MT level in MT+/+ mice was significantly increased by DMAA in a dose-dependent manner and there was no detectable amount of hepatic MT in untreated MT-/- mice.
CONCLUSION: DMAA treatment can lead to the induction of apoptosis and pathological damage in both types of mice. MT exhibits a protective effect against DMAA toxicity.
- Citation: Jia G, Gu YQ, Chen KT, Lu YY, Yan L, Wang JL, Su YP, Wu JCG. Protective role of metallothionein (I/II) against pathological damage and apoptosis induced by dimethylarsinic acid. World J Gastroenterol 2004; 10(1): 91-95
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/91.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.91
Arsenic is a metalloid that naturally occurs in soil, water, and air. Arsenicals are also non-biodegradable by-products during production of copper, lead, and other ores and coal consumption. Exposure to arsenic by food, drinking water, soil and air containing arsenic is widely existed in the world. Inorganic arsenicals are well known human carcinogens, specifically for the lung, liver, kidney, skin, bladder and other internal organs[1,2]. Dimethylarsinic acid (DMAA) is a major form of organic arsenic in the environment and the main metabolite of ingested inorganic arsenicals in most mammals, including humans[2-4]. DMAA itself can be used as herbicide and pesticide and also naturally exists in some seafood. Recent studies have revealed that DMAA is a genotoxic, multi-site promoter of carcinogenesis as well as a complete carcinogen in rodents[5-7], which provides a novel clue to investigate the mechanism of arsenicals in carcinogenesis.
Arsenicals, including DMAA, are moderately effective inducers of MT in mice and rats[8,9]. MTs, thiol-rich metal binding proteins, have been shown to be easily induced by oxidative stress and heavy metals and play an important role in homeostasis of essential metals, detoxication of heavy metals, scavenging reactive oxygen intermediates and preventing carcinogenesis as an endogenous defensive factor[10-15]. Especially to be mentioned, its capacity of scavenging hydroxyl and superoxide radicals is much more efficient than GSH, an established antioxidant[15]. Among the four major isoforms of identified MTs, MT-I and MT-II existing in all tissues examined, are the predominant forms in the livers. Recently Liu et al[16] reported that MT-I/II null mice were more sensitive than wild type mice to hepatotoxic and nephrotoxic effects of oral or injected inorganic arsenicals. Sakurai et al[17] reported that DMAA could induce apoptosis by reducing glutathione (GSH) in vitro. However, the effect of MT on induction of apoptosis and the main organic toxicity by DMAA in vivo remain elusive.
MT-I/II null (MT-/-) mice have been proved to be a good tool for studying MT’s normal function and the consequences of its deficiency[18]. In the present study, MT-I/II null (MT-/-) mice and the corresponding wild-type mice (MT+/+) were exposed to DMAA by oral injection, we investigated the pathological lesions and apoptosis in main target organs including the liver, lung and kidney of the mice, to elucidate the toxicity of DMAA and the ability of MT to modify DMAA toxicity.
Dimethylarsinic acid (purity 100 %) was purchased from Wako Pure Chemical Co. (Osaka, Japan). An in situ apoptosis detection kit (ApopTagTM) was purchased from Intergen Co. NY, USA.
MT null (MT-/-) mice whose MT-I and II genes had null mutation and wild type (MT+/+) mice provided kindly by Dr. A. Choo (Murdoch Institute for Research into Birth Defects, Royal Children’s Hospital, Australia), were of a mixed genetic background of 129 Ola and C57BL/6 strains. F1 hybrid mice were mated with C57BL/6 mice, and their offsprings were back-crossed to C57BL/6 for six generations. MT-/- and MT+/+ mice were obtained by mating of those heterozygous (MT-/+) mice.
MT-/- and MT+/+ mice were routinely bred in the vivarium of the National Institute for Environmental Studies (NIES, Japan). Microbiological and viral examinations were performed with regular quarantine procedures for more than one year, and we did not find either pathogenic infections or significant phenotypical abnormalities. Both strains of mice were housed in cages in ventilated animal rooms with a controlled temperature of 23 ± 1 °C, a relative humidity of 55 ± 10%, and a 12 h light/dark cycle. They were maintained on standard laboratory chow and tap water ad lib, and received humane care throughout the experiment according to the guidelines of the NIES. Eight-week-old female MT-/- and MT+/+ mice were assigned randomly in equal numbers to all groups (six mice for each treatment group). Fresh DMAA solution was prepared by dissolving it in sterilized water. The mice were administered DMAA (0-750 mg/kg) by oral gavage.
At 24 h after administration of DMAA, the lung, liver and kidney were collected from each mouse under diethyl ether anesthesia. Portions of tissues were fixed in 10% neutral formalin, processed by the standard histological techniques, and stained with hematoxylin and eosin for light microscopic examination. For TUNEL staining, sections (5 µm) were placed on poly-L-lysine precoated slides.
Apoptotic cells were detected with an apoptosis detection kit according to the manufacturer’s instructions. Briefly, the samples were incubated with digoxigenin-labeled dNTP in the presence of terminal deoxynucleotidyl transferase followed by peroxidase-conjugated anti-digoxigenin antibody. Nuclear staining of apoptotic cells was detected with 3’, 3’-diaminobenzidine followed by counterstaining of nuclei with methyl green. An apoptosis index (AI) was obtained by dividing the number of positive cells in the area observed[19].
MT (MT-I and MT-II isoforms) concentration in the liver was measured by radioimmunoassay using sheep anti-rat MT-I antiserum[20]. The detection limit of this method was 0.2 µg MT/g of tissue.
ANOVA with subsequent post hoc’s test was used as appropriate. All values were expressed as -χ±s. Differences were considered significant at P < 0.05.
In untreated MT-/- mice and the corresponding MT+/+ mice, the lung, liver and kidney showed normal morphology. Significant lesions were observed at doses of DMAA ranging from 375 to 750 mg/kg body weight in both types of mice. However, the pathological lesions in MT-/-mice were more severely widespread when compared to that in dose matched MT+/+ mice.
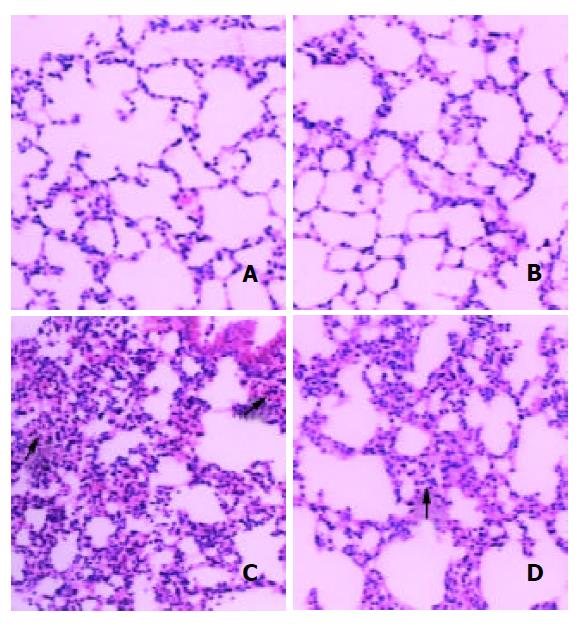
Changes including congestion, atelectasis and mild to moderate hemorrhages in the alveoli of the lungs were observed in MT-/- mice. Adequate air space in the alveoli was observed more frequent in MT+/+ mice compared to that of MT-/ - mice. Pulmonary capillary congestion could affect alveolar space, resulting in severe acute impairment of respiratory function. Capillary rupture led to leakage of red blood cells into the interstitium, as well as into the alveoli (Figure 1).
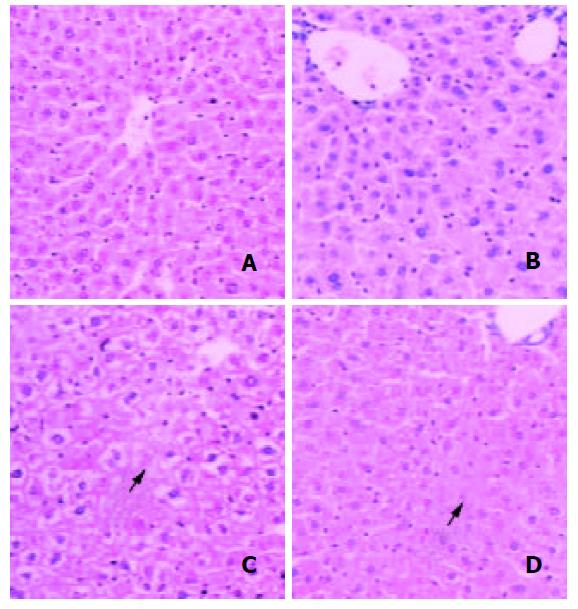
At 24 h after DMAA treatment, severe liver damages characterized by cellular cloudy swelling, paleness of cell cytoplasm, vacuolization of hepatocytes and a few areas of focal necrosis were found in MT-/- mice while a limited degree of changes was observed in dose matched MT+/+ mice livers (Figure 2).
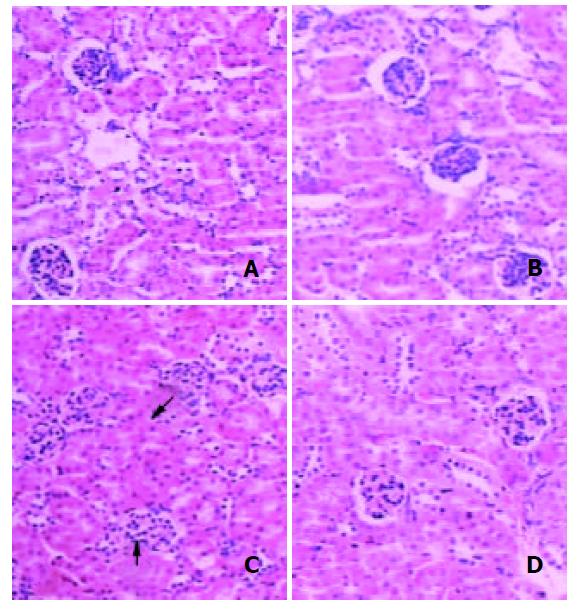
Histological changes in the kidney are shown in Figure 3. Treatment with DMAA produced swelling of glomerulus and its surrounding tubular tissue and urinary space compression in both types of mice.
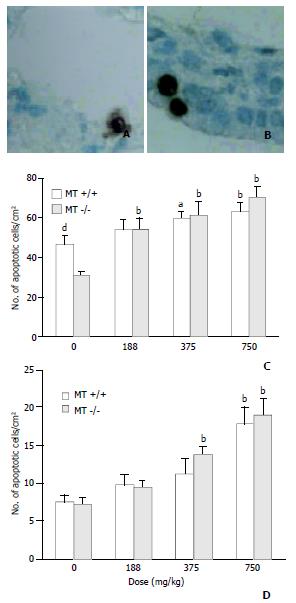
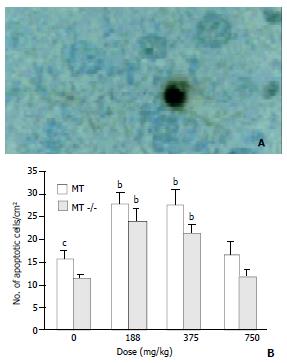
High induction of apoptotic cells in bronchial epithelial cells was observed in MT-/- mice treated with DMAA at 375 mg/kg body weight, however, the same changes were not observed in dose matched MT+/+ mice. At a high dose of 750 mg/kg body weight, the coincident increase of apoptotic cells was observed in both types of mice and no significant difference was observed between them.
In control group, the incidence of apoptotic cells in alveolar epithelial cells in MT+/+ mice was significantly higher than that in MT-/- mice, implying that MT+/+ mice might have a stronger ability to induce apoptosis than MT- / - mice. A significant increase of apoptotic cells occurred in MT-/- mice treated by 188 mg/kg DMAA, a relative low dose when compared with that in bronchial epithelial cells. However, no significant increase was observed in MT+/+ mice at the same dose of 188 mg/kg DMAA. With the increase of dose, high induction of apoptotic cells was observed in both types of mice (Figure 4).
Figure 5 shows that in control group, the incidence of apoptotic cells in MT+/+ mice was 156.33 ± 41.041/cm2, significantly higher than that in MT-/- mice. The incidence of apoptotic cells in the livers rose with the increase of dose in both types of mice. However, at the highest dose of 750 mg/kg, DMAA produced more necrotic cells rather than apoptotic cells observed by HE staining (Figure 2).
DMAA failed to induce remarkable apoptotic cells in the kidneys from both types of mice (data not shown).
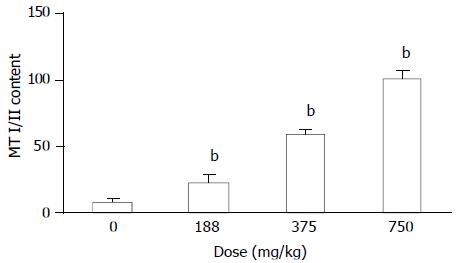
MT concentration was determined in the liver of MT+/+ mice and MT-/- mice treated with DMAA (Figure 6). Hepatic MT level in MT+/+ mice was significantly increased by DMAA in a dose-dependent manner. However, there was no detectable amount of hepatic MT in untreated MT-/- mice, and it could not be induced by DMAA.
DISCUSSION
The present study demonstrated that DMAA could produce pathological lesions in the lungs, livers and kidneys, and induce apoptosis in the lungs and livers. Most importantly, it is the first report to show that inability to produce MT-I/II in MT-/- mice caused an increased sensitivity to toxicity induced by DMAA.
Treatment with DMAA caused severe and wide spread lesions in MT-/- mice, whereas, these changes were much less severe in MT+/+ mice. It indicates that MT-/- mice were more sensitive to DMAA and MT played a protective role against the toxicities to main organs. Yamanaka[7] reported that DMAA in mice could be further metabolized and converted into dimethylarsine radicals and dimethyl arsenic peroxy radicals. Marked formation of 8-oxod G was observed in the lung and liver, which are the target organs for arsenic carcinogenesis. No increase in 8-oxod G levels was observed in the kidney. Meanwhile, MT was capable of scavenging hydroxyl and superoxide radicals and its capability of scavenging them was much more efficient[11,12,15]. In our study, the expression of MT (I/II) was induced by DMAA in a dose dependent manner in the livers of MT+/+ mice, no MT(I/II) was observed in MT-/-mice. Thus the reduction of lesions induced by DMAA in the main target organs of MT+/+ mice could be explained at least partly by MT reduction induced by DMAA, and the toxicity induced by DMAA could be explained partly by way of oxidative stress participation.
After a lethal damaging stimulation, two main structural routes of cell death might occur: apoptosis and necrosis. It has become apparent that the magnitude and type of injurious stimuli could determine whether a cell underwent death through apoptosis or necrosis. Severe damaging stimuli tended to result in necrosis, and lower grade damaged stimuli tended to cause apoptosis[21-30].
Recently, Sakurai et al reported that DMAA could induce apoptosis by reducing glutathione (GSH) in vitro[17]. However, the effect of MT on the induction of apoptosis by DMAA in vivo remains elusive. Furthermore, the perturbation of apoptosis has been thought to contribute to carcinogenesis either through enhanced initiation or progression[29-38]. In this study, the induction of apoptosis was detected by TUNEL in the lungs, livers and kidneys from both types of mice. In the lungs, a significant increase of apoptosis was observed in alveolar cells of MT-/- mice at a relative low level compared with that in bronchial cells, suggesting that alveolar cells were more sensitive than bronchial cells and MT had some protective role against the induction of apoptosis induced by DMAA at a relative low level. With the increase of dose, DMAA induced high levels of apoptosis in both types of mice and at the highest dose of 750 mg/kg, necrotic cells predominated over apoptosis in the livers, revealing the serious toxicity of DMAA at this dose. Since the kidney is the major organ for arsenic elimination and most of arsenicals could be rapidly eliminated through the kidney[1-3], renal cells are thus exposed to a major portion of the absorbed arsenical dose. However, the induction of apoptosis was not affected by DMAA, the underlying mechanism needs further investigations.
In conclusion, the present studies demonstrate that oral administration of DMAA can produce toxic response of the respiratory system, liver and kidney in both MT-/- and MT+/+ mice. The pathological effects are clearly pronounced in MT-/-mice. Intracellular MT appears to play an important role in preventing the toxic effects of DMAA.
We thank Dr. Sone Hideko and Masahiko Satoh (National Institute for Environmental Studies, Japan) for kindly providing the experiment materials. This work was supported in part by a Grant-in-aid for Scientific Research from the Ministry of Education, China and a grant from the Japan Science and Technology Agency, Japan.
Edited by Zhu LH and Wang XL
| 1. | Huff J, Chan P, Nyska A. Is the human carcinogen arsenic carcinogenic to laboratory animals. Toxicol Sci. 2000;55:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 492] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Goering PL, Aposhian HV, Mass MJ, Cebrián M, Beck BD, Waalkes MP. The enigma of arsenic carcinogenesis: role of metabolism. Toxicol Sci. 1999;49:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Braman RS, Foreback CC. Methylated forms of arsenic in the environment. Science. 1973;182:1247-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 221] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Yamamoto S, Wanibuchi H, Hori T, Yano Y, Matsui-Yuasa I, Otani S, Chen H, Yoshida K, Kuroda K, Endo G. Possible carcinogenic potential of dimethylarsinic acid as assessed in rat in vivo models: a review. Mutat Res. 1997;386:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kenyon EM, Hughes MF. A concise review of the toxicity and carcinogenicity of dimethylarsinic acid. Toxicology. 2001;160:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Yamanaka K, Takabayashi F, Mizoi M, An Y, Hasegawa A, Okada S. Oral exposure of dimethylarsinic acid, a main metabolite of inorganic arsenics, in mice leads to an increase in 8-Oxo-2'-deoxyguanosine level, specifically in the target organs for arsenic carcinogenesis. Biochem Biophys Res Commun. 2001;287:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kreppel H, Bauman JW, Liu J, McKim JM, Klaassen CD. Induction of metallothionein by arsenicals in mice. Fundam Appl Toxicol. 1993;20:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Albores A, Koropatnick J, Cherian MG, Zelazowski AJ. Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem Biol Interact. 1992;85:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Cherian MG, Howell SB, Imura N, Klaassen CD, Koropatnick J, Lazo JS, Waalkes MP. Role of metallothionein in carcinogenesis. Toxicol Appl Pharmacol. 1994;126:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol. 2000;59:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 606] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 12. | Viarengo A, Burlando B, Ceratto N, Panfoli I. Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol (Noisy-le-grand). 2000;46:407-417. [PubMed] |
| 13. | Cheng ML, Wu J, Wang HQ, Xue LM, Tan YZ, Ping L, Li CX, Huang NH, Yao YM, Ren LZ. Effect of Maotai liquor in inducing metallothioneins and on hepatic stellate cells. World J Gastroenterol. 2002;8:520-523. [PubMed] |
| 14. | Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650-653. [PubMed] |
| 15. | Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Sakurai T, Qu W, Sakurai MH, Waalkes MP. A major human arsenic metabolite, dimethylarsinic acid, requires reduced glutathione to induce apoptosis. Chem Res Toxicol. 2002;15:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci USA. 1993;90:8088-8092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 251] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Jia G, Tohyama C, Sone H. DNA damage triggers imbalance of proliferation and apoptosis during development of preneoplastic foci in the liver of Long-Evans Cinnamon rats. Int J Oncol. 2002;21:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Tohyama C, Shaikh ZA. Metallothionein in plasma and urine of cadmium-exposed rats determined by a single-antibody radioimmunoassay. Fundam Appl Toxicol. 1981;1:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Wu K, Zhao Y, Liu BH, Li Y, Liu F, Guo J, Yu WP. RRR-alpha-tocopheryl succinate inhibits human gastric cancer SGC-7901 cell growth by inducing apoptosis and DNA synthesis arrest. World J Gastroenterol. 2002;8:26-30. [PubMed] |
| 22. | Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol. 2002;8:36-39. [PubMed] |
| 23. | Shen ZY, Shen WY, Chen MH, Shen J, Cai WJ, Yi Z. Nitric oxide and calcium ions in apoptotic esophageal carcinoma cells induced by arsenite. World J Gastroenterol. 2002;8:40-43. [PubMed] |
| 24. | Shan CM, Li J. Study of apoptosis in human liver cancers. World J Gastroenterol. 2002;8:247-252. [PubMed] |
| 25. | Liu JW, Tang Y, Shen Y, Zhong XY. Synergistic effect of cell differential agent-II and arsenic trioxide on induction of cell cycle arrest and apoptosis in hepatoma cells. World J Gastroenterol. 2003;9:65-68. [PubMed] |
| 26. | Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435-437. [PubMed] |
| 27. | Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532-539. [PubMed] |
| 28. | Shen ZY, Shen J, Li QS, Chen CY, Chen JY, Yi Z. Morphological and functional changes of mitochondria in apoptotic esophageal carcinoma cells induced by arsenic trioxide. World J Gastroenterol. 2002;8:31-35. [PubMed] |
| 29. | Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2444] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 30. | Waalkes MP, Fox DA, States JC, Patierno SR, McCabe MJ. Metals and disorders of cell accumulation: modulation of apoptosis and cell proliferation. Toxicol Sci. 2000;56:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Manning FC, Patierno SR. Apoptosis: inhibitor or instigator of carcinogenesis. Cancer Invest. 1996;14:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Chen YX, Zhong XY, Qin YF, Bing W, He LZ. 15d-PGJ2 inhibits cell growth and induces apoptosis of MCG-803 human gastric cancer cell line. World J Gastroenterol. 2003;9:2149-2153. [PubMed] |
| 33. | Wang L, Li J, Li Q, Zhang J, Duan XL. Morphological changes of cell proliferation and apoptosis in rat jejunal mucosa at different ages. World J Gastroenterol. 2003;9:2060-2064. [PubMed] |
| 34. | Jiang YA, Luo HS, Zhang YY, Fan LF, Jiang CQ, Chen WJ. Telomerase activity and cell apoptosis in colon cancer cell by human telomerase reverse transcriptase gene antisense oligodeoxynucleotide. World J Gastroenterol. 2003;9:1981-1984. [PubMed] |
| 35. | Liu ZS, Tang SL, Ai ZL. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2003;9:1968-1971. [PubMed] |
| 36. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. Peroxisome proliferator-activated receptor gamma ligands inhibit cell growth and induce apoptosis in human liver cancer BEL-7402 cells. World J Gastroenterol. 2003;9:1683-1688. [PubMed] |
| 37. | Li JY, Wang XZ, Chen FL, Yu JP, Luo HS. Nimesulide inhibits proliferation via induction of apoptosis and cell cycle arrest in human gastric adenocarcinoma cell line. World J Gastroenterol. 2003;9:915-920. [PubMed] |
| 38. | Cheng ML, Wu J, Wang HQ, Xue LM, Tan YZ, Ping L, Li CX, Huang NH, Yao YM, Ren LZ. Effect of Maotai liquor in inducing metallothioneins and on hepatic stellate cells. World J Gastroenterol. 2002;8:520-523. [PubMed] |