Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.30
Revised: September 1, 1995
Accepted: September 15, 1995
Published online: October 1, 1995
AIM: To differentially diagnose gastric lymphoma by gastroendoscopic biopsy and clinicopathology.
METHODS: A retrospective review of 38 lymphoma cases diagnosed by gastroendoscopic biopsy in the period between 1984 and 1994 from gastroendoscopy files in the Department of Gastroenterology. The histology slides were examined retrospectively. Diagnostic criteria were discussed according to the new classification of non-Hodgkin’s lymphoma.
RESULTS: Of 53400 gastroendoscopy, 1672 were malignant neoplasms of which 38 were cases of the primary gastric lymphoma as diagnosed by both endoscopic findings and histological examination. A total of 22 men and 16 women, age 16 to 82 year, with a median of 47.7 year, were included in the study. The endoscopic evaluation found 12 cases of ulcerative, 11 cases of diffusely infiltrating, six cases of massive, four cases of large mucosal fold, and five cases of mixed type. The histological evaluation resulted in 34 cases of mucosa-associated lymphoid tissue lymphoma (89.5%), two cases with lymphoblastic type and two cases unclassified due to the crushed neoplastic cells.
CONCLUSION: These findings are present in about 90% of endoscopic biopsy specimens of low-grade gastric lymphoma. The majority of the cases of the primary low-grade gastric lymphoma have morphologic and clinical features that justify their inclusion in the category of low-grade lymphoma of mucosa-associated lymphoid tissue.
- Citation: Ji XL, Cheng YQ, Wang SQ. Gastroendoscopic biopsy diagnosis of mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol 1995; 1(1): 30-32
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/30.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.30
Primary gastric lymphoma is the most common extranodal lymphoma and accounts for 24% of all such tumors. In addition, non-neoplastic lymphoid hyperplasia is not uncommon in the stomach. The distinction between reactive lymphocytic proliferations and gastric lymphomas remains troublesome. This is reflected in numerous publications devoted to this problem[1]. It has been postulated that lymphomas involving mucosal surface arise from mucosa-associated lymphoid tissue and characterize a low-grade lymphoma called mucosa-associated lymphoid tissue lymphoma (MALToma), often with numerous reactive-appearing lymphoid follicles[2-4]. Therefore, the differential diagnosis of MALToma from reactive lymphoid hyperplasia remains difficult, especially by endoscopic biopsy with small pieces of tissue.
All gastric lymphomas diagnosed at the Department of Gastroenterology by gastroendoscopic biopsy during the period 1984-1994 were reviewed. Based on the new classification of lymphoid neoplasms[5], MALToma was confirmed if the following criteria were met: (1) invasion of epithelial structures resulting in lymphoepithelial lesions; (2) small lymphocytes, marginal zone cells and/or monocytoid B cells; and (3) infiltration of diffuse, perifollicular, interfollicular, or even follicular type due to colonization of reactive follicles. Tissue for light microscopy analysis was prepared in paraffin sections from 10% formalin and stained with hematoxylin and eosin.
From 1984 to 1994, a total of 53400 gastroendoscopy were performed, and 1672 cases of malignant gastric neoplasms were detected, including 38 cases of gastric lymphoma (2.3% of malignant tumors of the stomach). The patients (16 women and 22 men) ranged in age from 16 to 82 year, with a median of 47.7 year.
The endoscopic evaluation showed gastric lymphoma in various locations which included 10 cases in gastric antrum, six in gastric body, one in gastric fundus, 14 in gastric body and antrum, one in gastric body and fundus, four in gastric fundus, body and antrum, and two in gastric fundus and antrum. The endoscopic diagnosis showed five types of gastric lymphoma: ulcerative (12 cases), diffuse infiltrating (11 cases), massive (6 cases), large mucosal fold (4 cases), and mixed type (5 cases).
Based on the appearance of biopsy specimens, all 38 cases were gastric lymphomas of which 34 were MALToma (low-grade), two were lymphoblastic lymphoma (high grade) and two unclassified because of the crushed neoplastic cells.
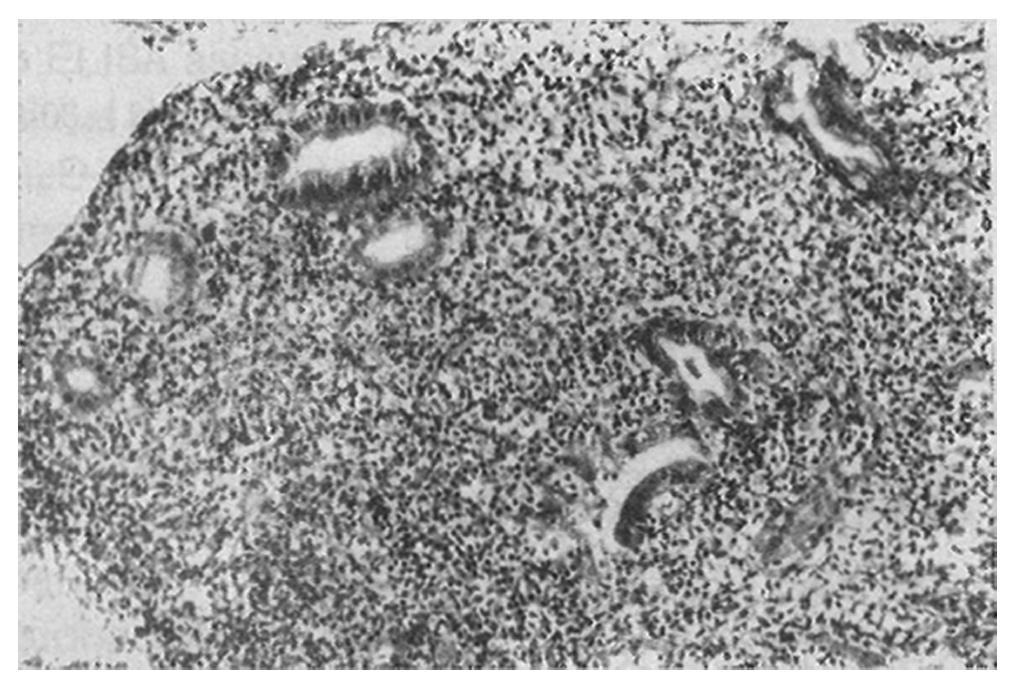
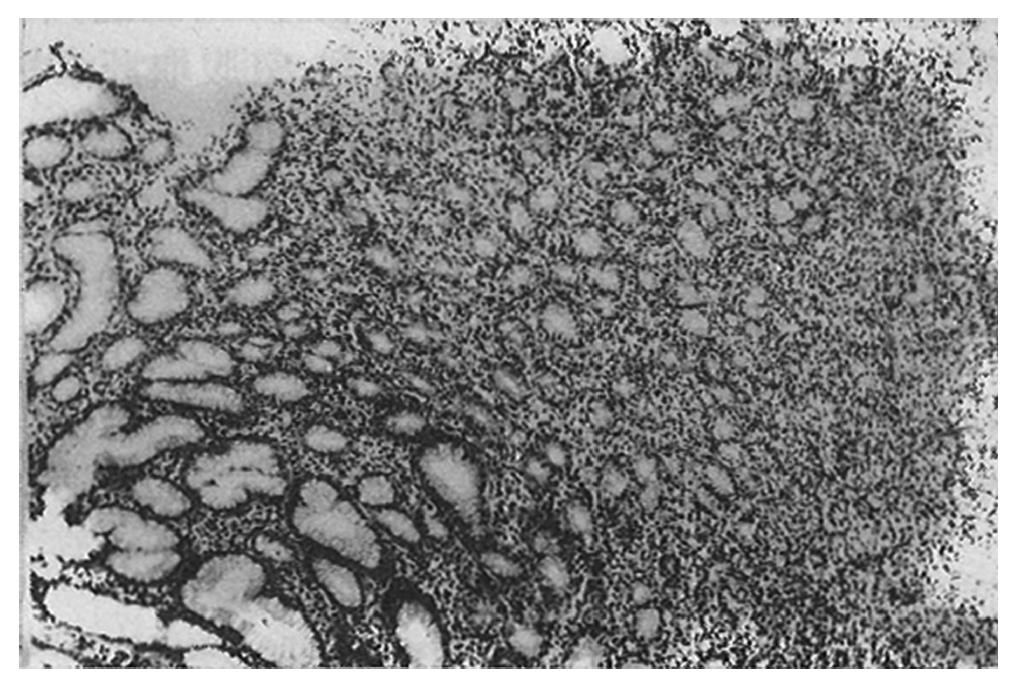
Initially, the low-grade MALToma was diagnosed as either suspected lymphoma (12/34, 35.3%) or as lymphoid hyperplasia (4/34, 11.8%). Of the 34 MALToma cases, 18 were seen with the diffuse infiltrating pattern of neoplastic lymphocytes (Figure 1), 12 with the interfollicular pattern (Figure 2), and four with the follicular pattern. Both of the latter two patterns were originally misdiagnosed as non-neoplastic lesions.
For the neoplastic lymphocytes, there were 21 cases with marginal zone cells, 12 with small lymphocytes, and five with monocytoid B-cells. All of them showed obvious lymphoepithelial lesions.
Several previous studies have reported the morphologic features of the primary gastric MALToma in the endoscopic biopsy specimens. The histopathological criteria for the diagnosis of gastric MALToma have largely been based on the analysis of partial gastrectomy specimens[4]. From a routine biopsy diagnosis by gastroendoscopy, it is very difficult to distinguish MALToma from reactive lymphoid hyperplasia on small pieces of tissue. In our series, 47.1% (16/34) of cases were misdiagnosed as non-neoplastic lesions. Nearly half of the cases had failed to have the right diagnosis of MALToma of the stomach.
Histologically, our results showed that the prominent lymphoepithelial lesions in the endoscopic biopsy specimens were one of the most important features for diagnosing MALToma of the stomach. The lymphoepithelial lesions were seen in 100% of the biopsied specimens. The diffuse infiltrating pattern was more appropriate than either interfollicular or follicular pattern for diagnosing MALToma of the stomach.
From our studies, we reflect that the key point for the diagnosis of MALToma is for the infiltrating lymphocytes to show homogeneity in the marginal zone cells, small lymphocytes, or monocytoid B cells. All of the three types of cells comprise low-grade lymphomas composed of a dense infiltrate with a superficial and peripheral plasma cell component.
Cytologically, neoplastic lymphocytes are characterized by cellular heterogeneity, including centrocyte-like cells (small, atypical cells with more abundant cytoplasm), monocytoid B cells, small lymphocytes, and plasma cells. Occasionally large cells (centroblast- or immunoblast-like cells) are present. If reactive follicles are present, the neoplastic cells will occupy the marginal zone and/or the interfollicular region. When the neoplastic lymphocytes take on a follicular pattern, it is called follicular colonization. Therefore, the diagnosis of MALToma of the stomach is not based on the infiltrating pattern, but on the cellular morphology.
In conclusion, we have demonstrated that nearly half of the MALToma cases of the stomach are misdiagnosed using the biopsy specimens. The diagnosis of gastric MALToma should be based on (1) dense lymphoid infiltrate with marginal zone cells, small lymphocytes or monocytoid B cells; or (2) prominent lymphoepithelial lesions. If a case is highly suspected for MALToma of the stomach, repeat biopsy should be performed. The presence of germinal centers, acute inflammation, crypt abscesses, and even associated Helicobacter pylori infection do not exclude lymphoma.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF
| 1. | Brooks JJ, Enterline HT. Gastric pseudolymphoma. Its three subtypes and relation to lymphoma. Cancer. 1983;51:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Isaacson PG, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology. 1987;11:445-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 492] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Isaacson PG, Spencer J, Finn T. Primary B-cell gastric lymphoma. Hum Pathol. 1986;17:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Chan JK, Banks PM, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Harris NL, Isaacson PG. A proposal for classification of lymphoid neoplasms (by the International Lymphoma Study Group). Histopathology. 1994;25:517-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |