Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.18
Revised: May 20, 1995
Accepted: July 20, 1995
Published online: October 1, 1995
AIM: To explore the biological effects of type I and III collagens in human hepatocellular carcinoma (HCC) and the relationship between the collagens and tumor behavior.
METHODS: The distribution of types I and III collagens was determined by immunohistochemistry in 25 specimens of human HCC and surrounding liver tissue, as well as six normal liver specimens. In addition, the expression of types I and III collagens were studied by in situ hybridization in nine HCC and two normal liver specimens. Collagen content in the tissues was calculated according to the theory of sterology.
RESULTS: The content of types I and III collagens was significantly lower in HCC than in the surrounding liver tissue. In addition, the collagen content was significantly lower in invasive/metastatic HCC tissue than in non-invasive/metastatic HCC tissue. However, collagen gene expression and protein synthesis were increased in HCC tissue.
CONCLUSION: The decrease in collagen content in HCC tissue likely resulted from collagen degradation. Collagens may be inhibitors of tumor invasion and metastasis.
- Citation: Wang YJ, Sun ZQ, Yu JJ, Xu XZ, Zhang X, Quan QZ. Biological effects of types I and III collagens in human hepatocellular carcinoma tissue. World J Gastroenterol 1995; 1(1): 18-20
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/18.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.18
Types I and III collagens regulate cell proliferation, migration, polarity and differentiation. In recent years, close attention has been paid to the relationships between collagens and tumor behavior[1]. The distribution, cellular origin and gene expression of interstitial collagen in hepatocellular cancer (HCC) tissues have been reported[2,3]. However, a quantitative study of collagen in HCC has not been performed, and the biological function of collagen in HCC is not clear. To explore the relationship between collagens and HCC behavior, a quantitative study of collagen was made according to the theory of sterology, and functions of collagen in HCC are discussed.
Specimens of liver tissue were obtained from 25 HCC patients by surgery. In each case, tissue was obtained from three places: the tumor, the surrounding liver tissue (SLT), and the juncture of the cancerous and non-cancerous tissue. In addition, normal liver tissue specimens were obtained from six persons who were killed in accidents. The samples were fixed in 10% buffered formalin and 5 μm-thick paraffin sections were cut and used for H&E staining and immunohistochemistry. Nine of the HCC specimens and 2 of the normal liver specimens were also frozen for in situ hybridization. A specific antibody against type I collagen was obtained as a gift from Yamamoto. A rabbit antibody against bovine procollagen III peptide was obtained from the Second Military Medical University. The ABC staining kit was purchased from Vector Laboratories. Recombinant DNA clones for in situ hybridization were a gift from Vuorio: PHCAL1 for proα-1 (I) collagen mRNA and PHFS3 for proα-1 (III) collagen mRNA. In the 25 cases of HCC (21 male, 4 female), the average age was 44.3 year (range 28-63 year). The serum AFP level was ≥ 400 μg/L in 10 of the HCC cases, and the serum was positive for HBsAg in 13 of the HCC cases.
Collagen quantitative study: Localization of type I and III collagens in the tissues was determined by immunohistochemistry[2]. Qa, the number of intersections of collagen and the test line (average of 5 fields per section and 3 sections per specimen), was calculated with the M168 multifunctional coherent test system recommended by Weibel using 400 × magnification. Length density was calculated as Jv = 2Qa/(Pc·K2·d2) and analyzed by Student’s t test.
In situ hybridization: cDNA probe preparation and in situ hybridization procedures in frozen sections were performed as previously described[3].
Histopathological observation was combined with gross specimen assessment and clinical data. According to Edmonson’s grading criteria, of the 25 cases of HCC, one was grade I, 11 were grade II, 12 were grade III and one was grade IV. Fifteen cases were accompanied by cirrhosis and eight cases were accompanied by chronic hepatitis. Tumors with invasion or/and metastasis were found in 18 cases. An entire envelope was formed in three cases.
In normal liver tissue, type I collagen was distributed in the portal connective tissue, the wall of central veins, and (non-continuously) in the sinusoidal wall. The distribution of type III collagen was identical to that of type I. In the SLT with chronic liver disease, deposition of types I and III collagen was found in connective tissue and in areas of inflammation and focal necrosis. Collagen was increased in the sinusoidal walls, which appeared wide and deep on immunohistochemical staining. The two types of collagen also had an identical distribution. However, within the tumor, the collagen content decreased significantly, surrounding the cancer nest and sometimes inside the nest. The tumors with a surrounding envelope had a significantly lower rate of invasion or/and metastasis.
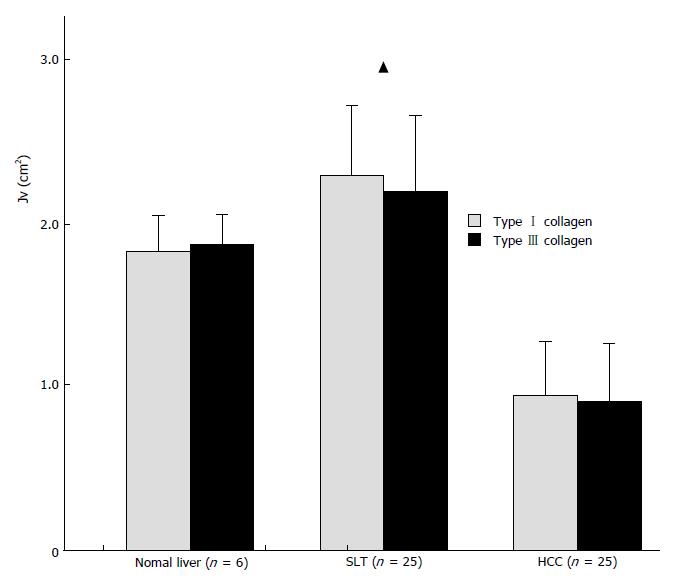
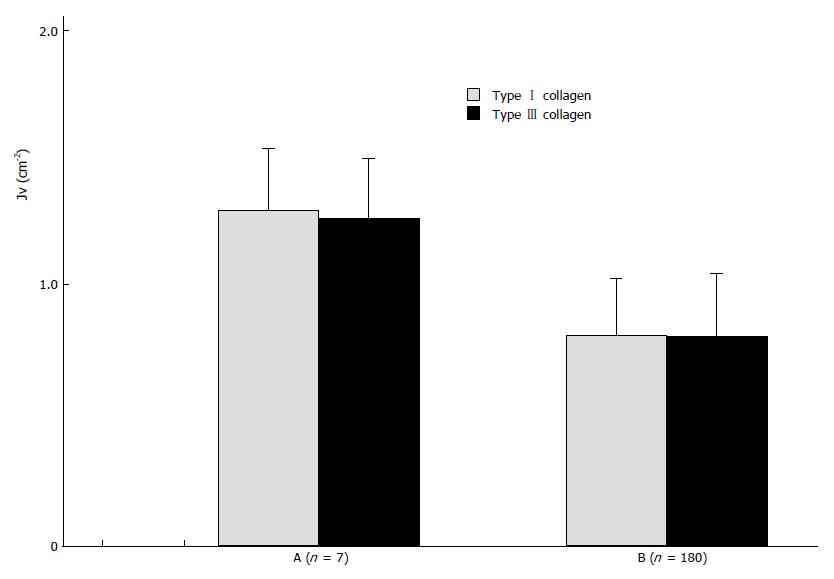
Collagen content was scaled by the length density of collagen (Jv). Collagen content in the tissues is shown in Figures 1 and 2. There was no significant difference in content between types I and III collagens.
In normal liver tissue, transcripts for types I and III collagens were not detected by in situ hybridization. In four cases of SLT (three with cirrhosis and one with persistent hepatitis), there were some weak signals in rare scattered mesenchymal cells. Interestingly, very intensive signals were found in many tumor cells in six of the nine cases of HCC.
According to the theory of sterology, we used the length density of collagen as a parameter for collagen content in HCC. We found that the content of types I and III collagen were much lower in HCC than in normal liver tissue and SLT (Figure 1). However, in situ hybridization showed that collagen gene expression is greater in HCC than in non-cancerous tissue. The rate of collagen protein synthesis in HCC has been shown to be increased, as well[4]. Therefore, we believe that the decrease in collagen content in HCC is due to excessive collagen degradation . Figure 2 shows that collagen content is significantly lower in cancer tissue with invasion or/and metastasis. It is possible that collagen degradation is closely linked to the tumor’s malignancy. Based on a series of studies, Liotta suggested that tumor cells can produce collagenase and thereby degrade the collagen matrix in HCC and its SLT. The local matrix degradation results in the formation of spatial pathway for invasion and metastasis. Additionally, stimulated by cell adhesive molecules and scatter factors, the tumor cells could scatter. The ability of hepatoma cells to produce collagenases may be an important factor affecting the invasion and metastasis of the tumor. Based on the present study, we believe that collagen degradation might promote tumor invasion and metastasis and that types I and III collagens might prohibit malignant behavior. This viewpoint is supported by the fact that a significantly lower invasive and metastatic tendency was present in tumors with an entire envelope. Collagens may act as a “screen” or perhaps regulate gene expression in the tumor cells[5]. As for the difference in collagen content between SLT and normal liver tissue, we believe the difference is related to the chronic liver disease present in the SLT.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF
| 1. | Guarino M, Christensen L. Immunohistochemical analysis of extracellular matrix components in synovial sarcoma. J Pathol. 1994;172:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Wang YJ, Dai YM, Zhang L. Immunohistochemical study on distribution of type I collagen and type III procollagen in tissues of human primary hepatocellular carcinoma (in Chinese). Pract J Cancer. 1993;8:65-68. |
| 3. | Wang YJ, Yang LS, Dai YM. Expression of collagen I gene in human primary liver carcinoma(in Chinese with English abstract). Linchuang Yu Shiyan Binglixue Zazhi. 1993;9:167-169. |
| 4. | Wang YJ, Quan QZ, Dai YM. Localization of P P in human primary liver cancer tissue by immunohistochemistry (in Chinese with English abstract). Linchuang Gandanbing Zazhi. 1994;10:78-79. |
| 5. | Iredale JP, Arthur MJ. Hepatocyte-matrix interactions. Gut. 1994;35:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |