Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.13
Revised: July 20, 1995
Accepted: September 1, 1995
Published online: October 1, 1995
AIM: To study the metabolic features of gastric cancer cells, the relationship between intestinal metaplasia or dysplasia and gastric cancer, and the relationship between LDH isoenzymes and the biological behavior of gastric cancer.
METHODS: The content and distribution of LDH isoenzymes LDH-5 and LDH-H in 60 cases of gastric cancer were examined by immunohistochemistry and immunoelectron microscopy.
RESULTS: The content of LDH-5 was significantly higher in gastric cancer cells than in chief cells, surface epithelium and pyloric glandular epithelium, but was similar between gastric cancer cells and parietal cells. The content of LDH-H was significantly lower in gastric cancer cells than in parietal cells, but was similar between gastric cancer cells, chief cells and surface epithelium. The product of LDH-5 but not LDH-H was mainly distributed in the cytoplasmic matrix in the cancer cells. The content of LDH-5 in intestinal metaplasia and dysplasia was significantly higher than in the pyloric glandular epithelium, but was similar to the content in gastric cancer cells. The content of LDH-H was significantly higher in intestinal metaplasia and dysplasia than in both the pyloric glandular epithelium and gastric cancer cells.
CONCLUSION: Increased LDH in gastric cancer cells resulted mainly from increased levels of LDH-5. The LDH-5-mediated elevation in lactate levels could decrease the pH, thereby activating acid hydrolase and indirectly promoting the invasion and spread of gastric cancer cells. Intestinal metaplasia and dysplasia might be an intermediate phase between normal gastric mucosa and gastric cancer.
- Citation: Song YL, Yang GL, Dong YM. Immunohistochemical study of lactate dehydrogenase isoenzymes in gastric cancer. World J Gastroenterol 1995; 1(1): 13-17
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/13.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.13
Studies of lactate dehydrogenase (LDH) isoenzymes in gastric cancer tissues have generally been carried out using biochemical methods[1-3]. Immunohistochemical studies, which lack systematization, have scarcely been reported in the literature[4]. In the present study, the content and distribution of LDH isoenzymes LDH-5 and LDH-H in normal gastric mucosa, intestinal metaplasia, dysplasia and various kinds of gastric cancer were analyzed using immunohistochemistry (IHC) and immunoelectron microscopy. The metabolic features of gastric cancer cells and the relationship between LDH isoenzyme content and the biological behavior of gastric cancer are discussed.
Surgically resected specimens from 60 cases of gastric cancer (including 4 cases of early cancer), as well as normal mucosa > 2 cm from the tumor and transitional tissue between the tumor and normal mucosa, were fixed in 10% neutral-buffered formalin. After embedding the tissues in paraffin wax, 5 μm-thick serial sections were cut and stained by hematoxylin and eosin (H&E) or IHC. Antibodies specific for LDH-5 and LDH-H (both from Sigma, St. Louis, MO, United States) were diluted 1:100 and 1:25, respectively, and detected with an ABC kit from Vector Laboratories (Burlingame, CA, United States). Both negative and positive controls were used for all cases. Negative control slides were included by substituting the primary antibody with PBS, and skeletal muscle and myocardium were used as positive control tissues for LDH-5 and LDH-H, respectively.
Specimens from nine of the 60 cases of gastric cancer were cut in ultrathin sections using a pre-embedding immunoelectron microscopic technique in our laboratory[5].
Referring to Berry’s grading[6], the IHC staining patterns were scored according to the relative intensity and extent of immunoperoxidase staining. The intensity of staining was scored as negative (0), weak yellow (1), yellow-brown (2) and dark brown (3). The extent of staining was scored as negative (0), less than one-third of the cells stained (1), one-third to two-thirds of the cells stained (2), and more than two-thirds of the cells stained (3). Then the scores were added and the cases were divided into five grades: no points/not stained at all (-), 2-3 points (±), 4 points (+), 5 points (+ +) and 6 points (+++). Grades (-) and (±) were regarded as negative staining, and grades (+) to (+++) as positive staining.
The IHC grades from the tissues were compared by Rank sum test, and the rates of staining were compared by Chi square test. P < 0.05 was considered significant.
The content and distribution of LDH-5 and LDH-H in normal gastric mucosa, precancerous lesions and gastric cancer cells are shown in Table 1, Table 2, Table 3, Table 4.
The content and distribution of LDH-5 and LDH-H in the normal gastric body and antral epithelium (including surface epithelium, parietal cells, chief cells and pyloric glandular epithelium) were mainly observed in the present study. Staining in parietal cells was diffusely distributed in the cytoplasm, whereas staining in chief cells was confined to the supranuclear cytoplasm. The staining intensity and patterns of LDH-5 and LDH-H varied among the cell types. Parietal cells and chief cells were dominated by LDH-H, whereas surface epithelium and pyloric glandular epithelium were dominated by LDH-5. Parietal cells showed the strongest staining for LDH-H and LDH-5 in normal gastric epithelium. The staining intensity of LDH-H and LDH-5 in the surface epithelium and pyloric glandular epithelium were not significantly different. The positive product of LDH-5 and LDH-H in parietal cells, being mostly granular, was located in the cytoplasmic matrix around the mitochondria and on the microvilli of intracellular canaliculi. In contrast, the positive product of LDH-5 and LDH-H in chief cells was located in the cytoplasmic matrix around the zymogen granules and endoplasmic reticulum, and sometimes on the membrane of endoplasmic reticulum.
Intestinal metaplastic epithelium showed moderately strong staining for LDH-5 and LDH-H, whereas pyloric glandular epithelium showed weaker staining for both isoenzymes. The intestinal metaplastic epithelium was dominated by LDH-5. The staining product was located in the supranuclear cytoplasm of absorptive cells and in the cytoplasm around mucin of goblet cells.
Dysplastic epithelium also showed moderately strong staining for LDH-H and LDH-5, and pyloric glandular epithelium also showed weaker staining for both isoenzymes. The dysplastic epithelium was dominated by LDH-5. The staining product was primarily located in the supranuclear cytoplasm of dysplastic epithelium.
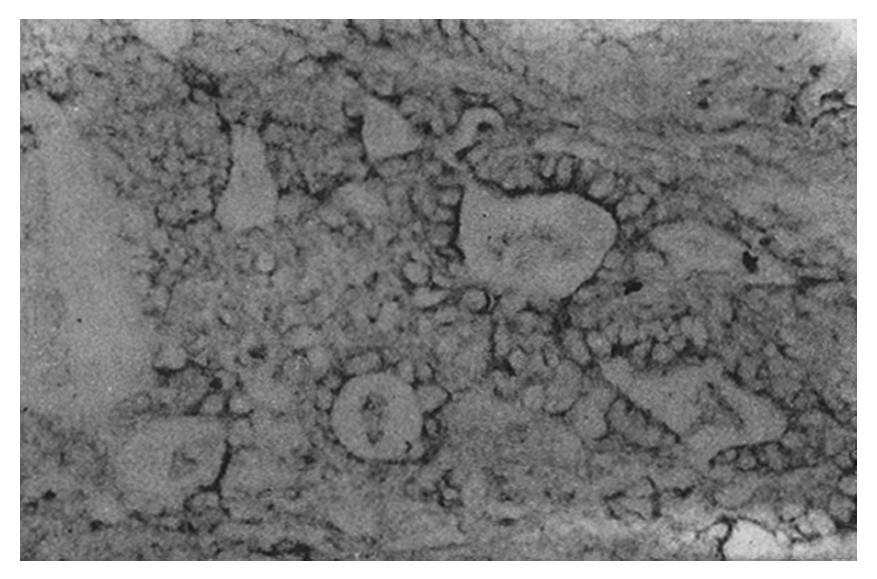
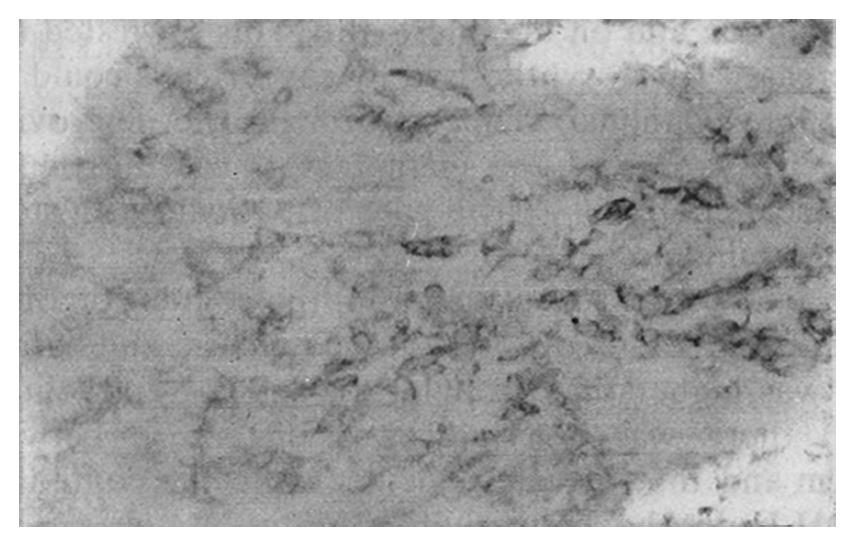
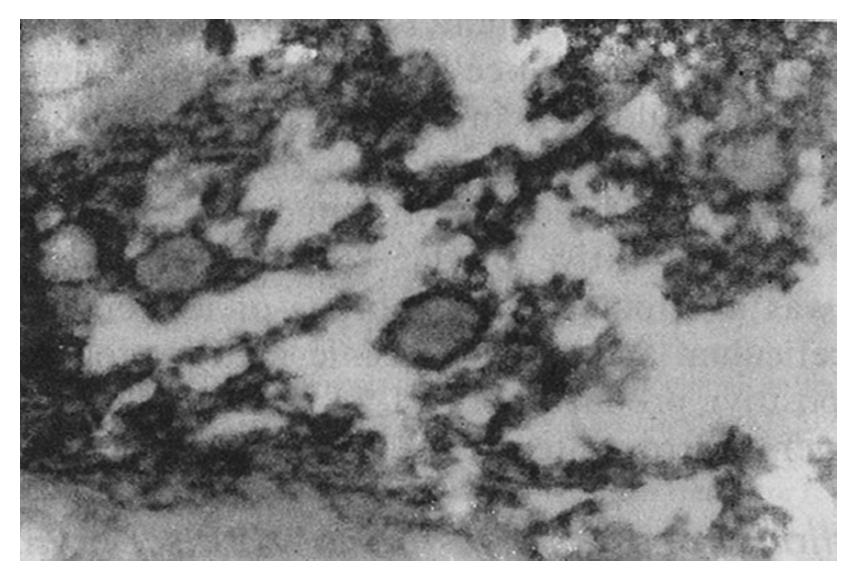
The staining intensity of LDH-5 was stronger in gastric cancer cells than in surface epithelium, pyloric glandular epithelium and chief cells. There was no significant difference in LDH-5 staining between gastric cancer cells and parietal cells, intestinal metaplastic epithelium and dysplastic epithelium. The staining intensity of LDH-H was weaker in gastric cancer cells than in intestinal metaplastic epithelium, dysplastic epithelium and parietal cells. There was no significant difference in LDH-H staining between gastric cancer cells and surface epithelium, pyloric glandular epithelium and chief cells. Staining in cancer cells was dominated by LDH-5. The rate of LDH-5 and LDH-H staining in 27 cases of gastric body cancer was 88.9% (24/27) and 56.3% (9/16), respectively, whereas the rate of LDH-5 and LDH-H staining in 33 cases of gastric antral cancer was 93.9% (31/33) and 20.0% (3/15), respectively . No significant difference existed between the rates of staining in body cancer cells and antral cancer cells. Although the relative intensities of the two antibodies varied, LDH-5 and LDH-H generally exhibited the same staining patterns. The mAbs stained well-differentiated gastric adenocarcinoma primarily in the luminal aspect or in the apical cytoplasm of cancerous glands, and in the cytoplasm of some cancer cells (Figure 1). In poorly differentiated adenocarcinoma, the staining was mostly distributed in the cytoplasm (Figure 2). In signet-ring cell carcinoma the staining was distributed in a ring round the cell membrane. In addition, the heterogeneity of LDH-5 and LDH-H in gastric cancer cells was apparent both in the relative intensity of staining and the distribution of staining, i.e., different intensities of staining and different types of distribution (polar and diffuse) were seen in different areas of the same section. By electron microscopy, the positive product of LDH-5 in cancer cells of tubular adenocarcinoma appeared as coarse, large granules distributed in the cytoplasmic matrix primarily around mitochondria and the endoplasmic reticulum (Figure 3) and partly near the cell membrane and on the microvilli. In contrast, the positive product of LDH-5 in poorly differentiated adenocarcinoma appeared as fine, small granules diffusely distributed in the cytoplasmic matrix. The positive product of LDH-H in cancer cells was distributed less on the membrane of the endoplasmic reticulum and in the cytoplasmic matrix around it. The positive product of LDH-5 in cancer cells showed stronger electron intensity than did the positive product of LDH-H.
There was no significant correlation between the positive rate of LDH-5 and LDH-H staining in gastric cancer and the cancer’s histological types, degrees of differentiation, size, clinical stages, manner of growth and lymph node metastases.
LDH is an important oxidoreductase in glycolysis, catalyzing the transition between pyruvate and lactate. LDH is actually a group of enzymes that are tetramers composed of two types of subunits (H and M). Differences in the proportions of M and H subunits in LDH can yield five types of isoenzymes (LDH-1 through LDH-5). Since the LDH isoenzymes possess different molecule constitutions, functional differences exist among them to some extent. LDH-5 easily converts pyruvate into lactate, which benefits anaerobic metabolism, whereas LDH-1 converts lactate into pyruvate, which can be oxidized in the citric acid cycle and thus benefits aerobic metabolism. In the present study, antibodies against LDH-5 and the H subunit (present in LDH-1 through LDH-4) were used in an IHC survey. We found that the LDH isoenzyme patterns varied with different kinds of cells in normal gastric epithelium, consistent with a previous study[4]. LDH-H dominated LDH-5 in parietal cells and chief cells, but LDH-5 dominated LDH-H in surface epithelium and pyloric glandular epithelium. Parietal cells showed the strongest staining for both LDH-5 and LDH-H, which is not surprising given the cells’ active acid secreting activity[7]. The mechanisms of different LDH isoenzymes in various kinds of cells warrant further investigation.
Normal gastric epithelium is composed of many kinds of cells with varying structure and functions, and the histological origin of gastric cancer varies by cell type. It would be inappropriate if normal gastric mucosa was used as control without indicating its corresponding site in the stomach. In this study, we compared gastric cancer cells from different sites with the corresponding normal gastric epithelium in order to elucidate the metabolic features of the gastric cancer cells. We found that the content of LDH-5 was higher in gastric cancer cells than in chief cells, surface epithelium and pyloric glandular epithelium. The content of LDH-H in gastric cancer cells was lower than that in parietal cells and similar to the content in chief cells, surface epithelium and pyloric glandular epithelium. In the cancer cells, LDH-H was dominated by LDH-5. By electron microscopy, the positive product of LDH-5 was found intensely in coarse granules that were distributed in the cytoplasmic matrix around the mitochondria and endoplasmic reticulum. In contrast, the positive product of LDH-H could only be seen diffusely in the cytoplasmic matrix. Therefore, at both the cellular and ultrastructural level, our data indicate that the increased LDH in gastric cancer cells[2,3] is mainly due to a selective increase in LDH-5. This change in the gastric cancer cells would increase glycolysis and thereby rapidly generate energy for proliferation. The positive product of LDH-5 in the cancer cells was also seen in the cytoplasmic matrix near the cell membrane and on the microvilli. This location suggests that the newly-synthesized LDH-5 could enter into the glandular lumen through the microvilli, consistent with the increased levels of LDH-5 found in the gastric juice of patients with stomach carcinoma.
In intestinal metaplasia and dysplasia, the content of LDH-5 was higher in the surface epithelium than in the pyloric glandular epithelium, whereas the content of LDH-H was higher in the epithelium than in both the pyloric glandular epithelium and the cancer cells. LDH-H was dominated by LDH-5. Thus, LDH isoenzymes in intestinal metaplastic epithelium and dysplastic epithelium are similar to, though not the same as, those in cancer cells. Intestinal metaplasia and dysplasia could be a borderline lesion between gastric cancer and normal gastric mucosa; though increased LDH-5 content might not be tumor-specific, it could be closely related to the proliferative activity of the cancer cells[4]. Our LDH-H results are not consistent with Carda-Abella’s biochemical result[2], perhaps due to factors influencing the biochemical result[8] and the use of different counting methods.
Few studies have probed the relationship between gastric cancer cell LDH isoenzyme content and biological behavior[2,3,9]. Our study showed there was no significant correlation between the expression of LDH-5 and LDH-H in gastric cancer cells and their biological behavior. This result might be due to many factors, including the degrees of cellular carcinogenesis, invasion or metastasis. Increased levels of LDH, especially LDH-5, might increase glycolysis in cancer cells, raising lactate concentrations in the tumor and adjacent tissue. The subsequent decrease in pH by acid hydrolase might then promote invasion and metastasis of gastric cancer cells[9].
Presented at V Chinese Conference on Gastric Cancer, Shanghai, 8th November 1993.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF
| 1. | Hirata RD, Hirata MH, Strufaldi B, Possik RA, Asai M. Creatine kinase and lactate dehydrogenase isoenzymes in serum and tissues of patients with stomach adenocarcinoma. Clin Chem. 1989;35:1385-1389. [PubMed] |
| 2. | Carda-Abella P, Perez-Cuadrado M, Mate-Jimenez J. LDH isoenzyme patterns in human gastric mucosa with precancerous changes. Cancer. 1978;42:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Wang ZS. [Activity of lactic dehydrogenase and its isoenzyme in intestinal metaplasia of the gastric mucosa, early and advanced gastric carcinoma]. Zhonghua Zhongliu Zazhi. 1985;7:260-262. [PubMed] |
| 4. | Pan LX, Xu JN, Isaacson PG. Cellular H- and M-type lactate dehydrogenase (LDH) isoenzymes and tumor diagnosis--an immunohistochemical assessment. J Pathol. 1991;163:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Dong YM, Wu JF, Yang GL, Chen XL, Xu AL, Zhou L. The application of immunoelectron microscopic technique in the location of CEA in gastric cancer. Linchuang Yu Shiyan Binglixue Zazhi. 1987;3:42-43. |
| 6. | Berry N, Jones DB, Smallwood J, Taylor I, Kirkham N, Taylor-Papadimitriou J. The prognostic value of the monoclonal antibodies HMFG1 and HMFG2 in breast cancer. Br J Cancer. 1985;51:179-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Elkeles RS, Tavill AS(Compile). Wang SY(main translator). Biochemicl aspects of human disease. Beijing: The People’s Health Publication 1988; 175-176. |
| 8. | Carda-Abella P, Perez-Cuadrado S, Lara-Baruque S, Gil-Grande L, Nuñez-Puertas A. LDH isoenzyme patterns in tumors, polyps, and uninvolved mucosa of human cancerous colon. Cancer. 1982;49:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Dai DQ, Chen JQ, Ren CS, Zhang WF. The relation of |a2glucuronidase, acid phosphatase and lactate dehydrogenase with gastric cancer. Zhongguo Yike Daxue Xuebao. 1991;20:131-135. |