Copyright
©The Author(s) 2019.
World J Gastroenterol. Jan 21, 2019; 25(3): 330-345
Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.330
Published online Jan 21, 2019. doi: 10.3748/wjg.v25.i3.330
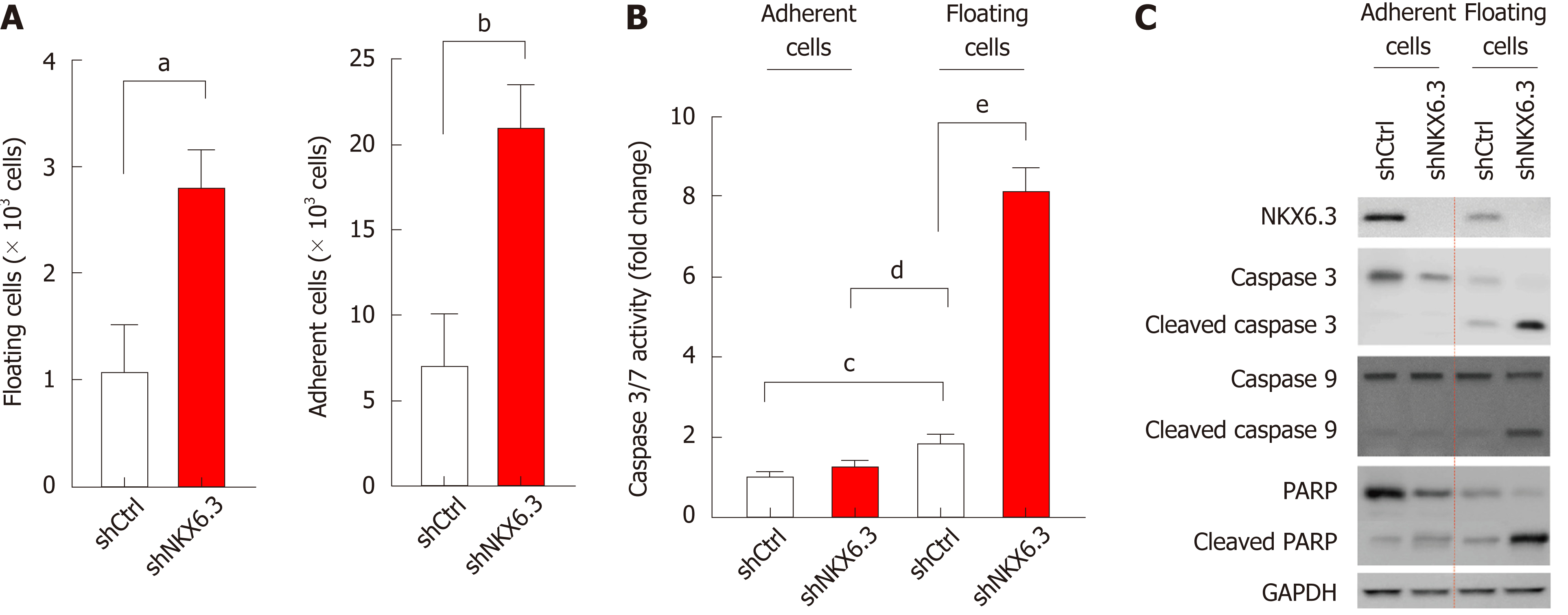
Figure 1 NKX6.
3 inhibits both cell proliferation and apoptotic cell death in gastric epithelial cells. A: NKX6.3 depletion in HFE-145shNKX6.3 cells increased both adherent and floating cell populations. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (aP < 0.005). B: Caspase 3/7 activity was significantly increased in floating HFE-145shNKX6.3 cells. Shown are the means ± SEM from three experiments. Superscript letter significant difference (bP < 0.0001). C: In Western blot analysis, expression of cleaved forms of caspase-3, -9, and poly ADP ribose polymerase (PARP) was increased in floating HFE-145shNKX6.3 cells.
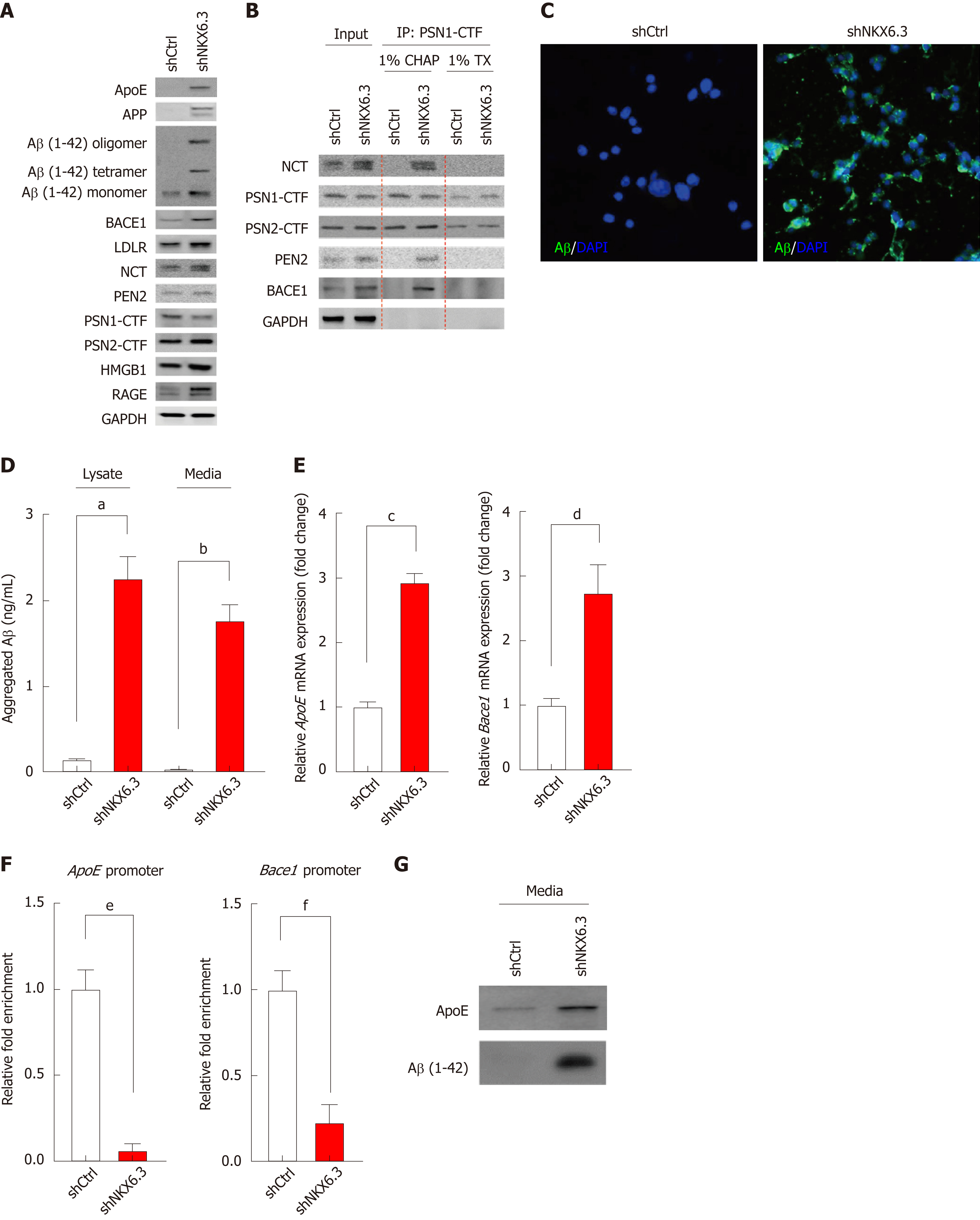
Figure 2 NKX6.
3 inhibits amyloid β accumulation by regulating apolipoprotein E, β-, and γ-secretase. A: Western blot analysis showing increased expression of apolipoprotein E (ApoE), amyloid precursor protein (APP), amyloid β (Aβ), β-secretase 1 (Bace1), low-density lipoprotein receptor (LDLR), nicastrin (NCT), high mobility group box1 (HMGB1), and receptor for advanced glycosylation end product (RAGE) proteins but decreased expression of presenilin 1 (PSN1)- C-terminal (CTF) protein in NKX6.3 depleted HFE-145 cells. Interestingly, HFE-145shCtrl cells expressed only Aβ monomer whereas NKX6.3 depleted HFE-145shNKX6.3 cells induced production of oligomers of Aβ and increased expression of Aβ monomer. B: In immunoprecipitation assay, γ-secretase complex including PSN1-CTF and 2-CTF, NCT, and presenilin enhancer 2 (PEN2) was stably formed, binding to Bace1 protein only in NKX6.3 depleted HFE-145shNKX6.3 cells. C: Immunofluorescence images showing strong expression of Aβ protein in the cytoplasm of NKX6.3 depleted HFE-145shNKX6.3 cells. D: In Aβ42 enzyme-linked immunosorbent assay, NKX6.3 depletion significantly increased concentrations of aggregated Aβ in both cell lysate and culture media. Shown are the means ± SEM from three experiments. Superscript letter significant difference (aP < 0.0001). E: Increased mRNA expression of ApoE and Bace1 genes was detected in NKX6.3 depleted HFE-145shNKX6.3 cells. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (bP < 0.005 and cP < 0.0001). F: In chromatin immunoprecipitation assay, the binding capacity of NKX6.3 to promoter regions of ApoE and Bace1 genes was dramatically decreased. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (dP < 0.005 and eP < 0.0001). G: When we treated HFE-145shCtrl and HFE-145shNKX6.3 cells with Aβ 1-42 recombinant protein (rAβ 1-42), expression levels of ApoE and oligomeric forms of Aβ were makedly increased in NKX6.3 depleted HFE-145shNKX6.3 cells. CHAP: Chaps buffer; TX: Triton-100; ApoE: Apolipoprotein E; APP: Amyloid precursor protein; Aβ: Amyloid β; Bace1: β-secretase 1; LDLR: Low-density lipoprotein receptor; NCT: Nicastrin; HMGB1: High mobility group box1; RAGE: Receptor for advanced glycosylation end product; PSN1: Presenilin 1; CTF: C-terminal; PEN2: Presenilin enhancer 2; rAβ 1-42: Aβ 1-42 recombinant protein.
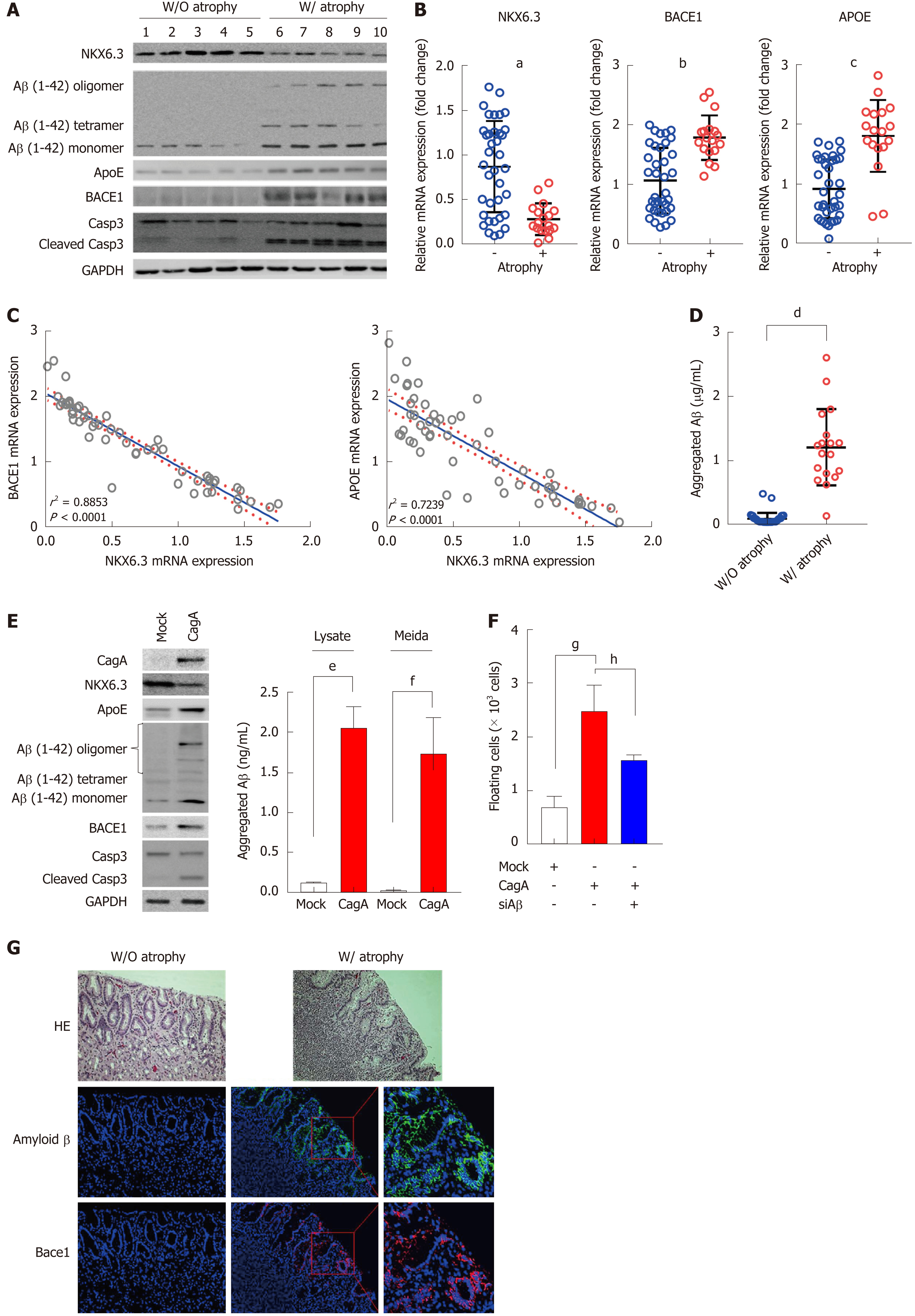
Figure 3 Amyloid β oligomerization is strongly associated with gastric mucosal atrophy.
A: In Western blot analysis, amyloid β (Aβ) oligomers were expressed only in gastric mucosae with atrophy. Expression of apolipoprotein E (ApoE), β-secretase 1 (Bace1), and cleaved form of caspase-3 were increased in atrophic gastric mucosae with reduced NKX6.3 expression. B and C: Expression levels of ApoE and Bace1 mRNAs were significantly higher in gastric mucosae with atrophy (B), showing inverse correlations with NKX6.3 expression (P < 0.0001, C). Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (aP < 0.0001, bP < 0.0001 and cP < 0.0001). D: In Aβ42 enzyme-linked immunosorbent assay, concentrations of aggregated Aβ were significantly increased in gastric mucosae with (W/) atrophy (1.20 ± 0.60 μg/mL) compared to without (W/O) atrophy (0.09 ± 0.09 μg/mL). Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (dP < 0.0001). E: Western blot analysis showing decreased expression of NKX6.3 and increased expression of ApoE, Aβ oligomer, Bace1, and cleaved caspase-3 proteins in CagA transfected HFE-145 cells. In addition, CagA significantly increased concentrations of aggregated Aβ in both cell lysate and culture media. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (eP < 0.005 and fP < 0.0001). F: Knock-down of Aβ inhibited CagA-induced floating cells populations. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (gP < 0.005 and hP < 0.0001). G: Immunofluorescence analysis showing expression of Aβ oligomer and Bace1 only in gastric mucosae with atrophy, but not in gastric mucosa without atrophy. Right panel is a higher magnification of the Aβ oligomer and Bace1 in the inlet in gastric mucosae with atrophy. ApoE: Apolipoprotein E; Aβ: Amyloid β; Bace1: β-secretase 1; W/: With atrophy; W/O: Without atrophy.
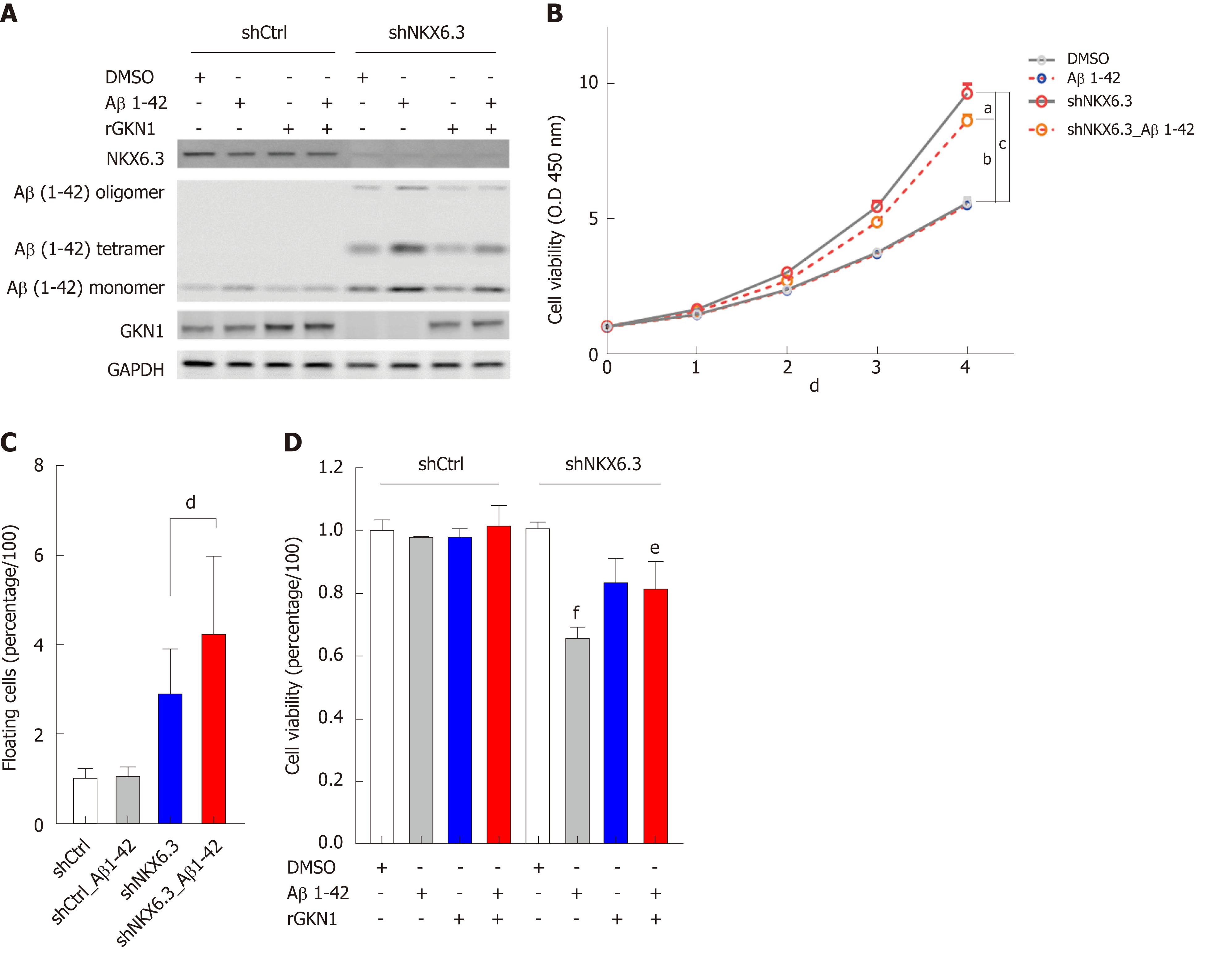
Figure 4 Amyloid β induces cell death in gastric epithelial cells.
A: Treatment with recombinant amyloid β (rAβ) 1-42 produced oligomeric forms of Aβ in HFE-145shNKX6.3 cells, but not in HFE-145shCtrl cells. Recombinant gastrokine 1 (rGKN1) partially reduced expression of Aβ oligomers. B: Treatment with rAβ 1-42 did not affect viability of HFE-145shCtrl cells, although it significantly decreased viability of HFE-145shNKX6.3 cells in a time dependent manner. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (aP < 0.05, bP < 0.005 and cP < 0.0001). C: Floating cell population was markedly increased in HFE-145shNKX6.3 cells treated with rAβ 1-42. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (dP < 0.001). D: In HFE-145shNKX6.3 cells, treatment with rAβ 1-42 for 72 hrs decreased cell viability while treatment with rGKN1 revoked the effect of rAβ on cell viability. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (eP < 0.05 and fP < 0.005). rAβ: Recombinant amyloid β; rGKN1: Recombinant gastrokine 1; Aβ: Amyloid β.
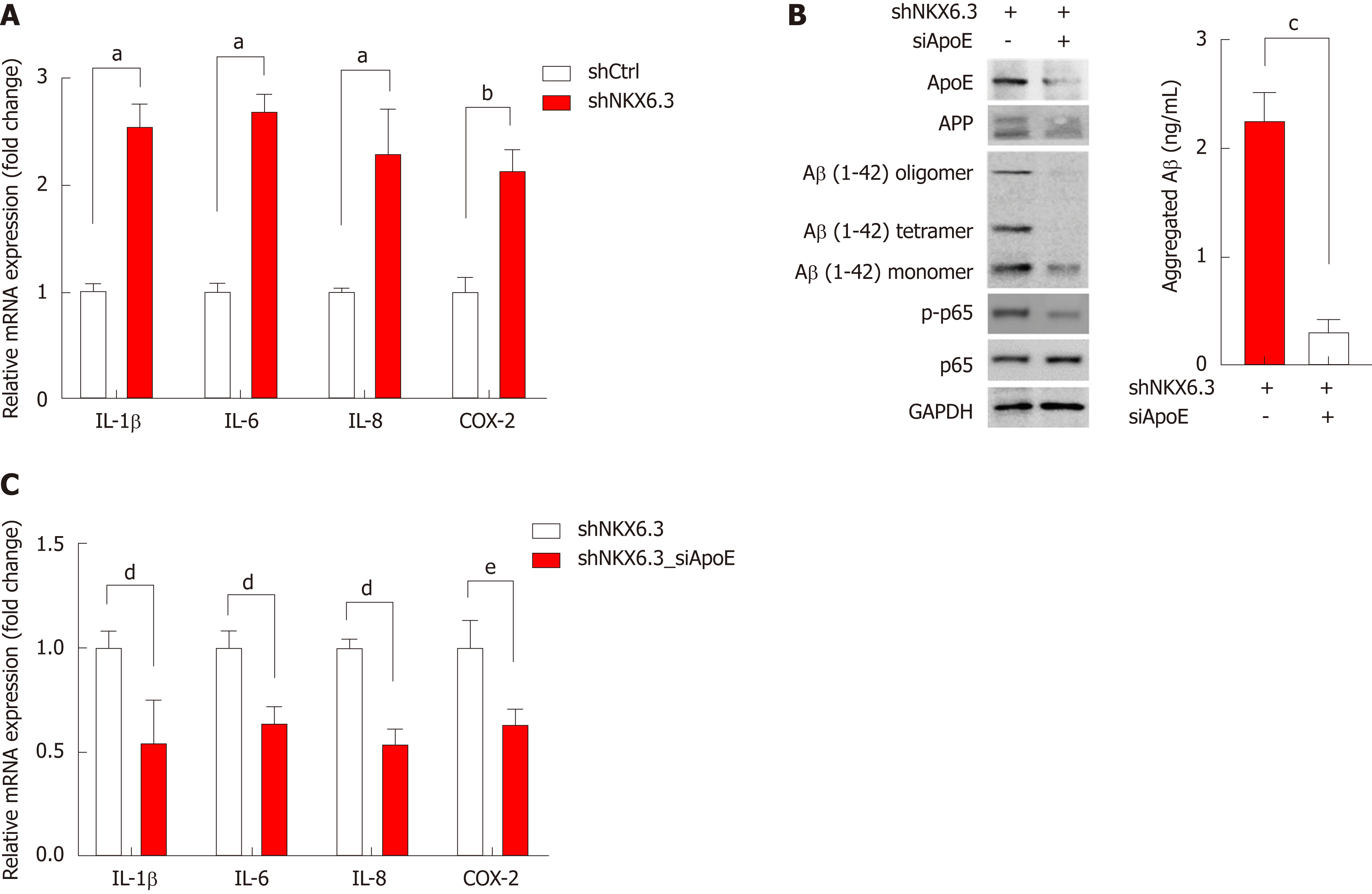
Figure 5 NKX6.
3 depletion induces inflammatory cytokine expression in gastric epithelial cells. A: NKX6.3 depletion in HFE-145shNKX6.3 cells dramatically increased expression of inflammatory cytokines, including interleukin (IL)-1β, IL-6, and IL-8, and cyclooxygenase-2. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (aP < 0.005 and bP < 0.001). B and C: In HFE-145shNKX6.3 cells, silencing of apolipoprotein E (ApoE) with siApoE markedly inhibited oligomerization and aggregation of amyloid β and expression of nuclear factor kappa B p-p65 and inflammatory cytokines. Shown are the means ± SEM from three experiments. Superscript letter represents significant difference (cP < 0.05, dP < 0.005 and eP < 0.0001). IL: Interleukin; COX-2: Cyclooxygenase-2; ApoE: Apolipoprotein E; Aβ: Amyloid β; NF-κB: Nuclear factor kappa B.
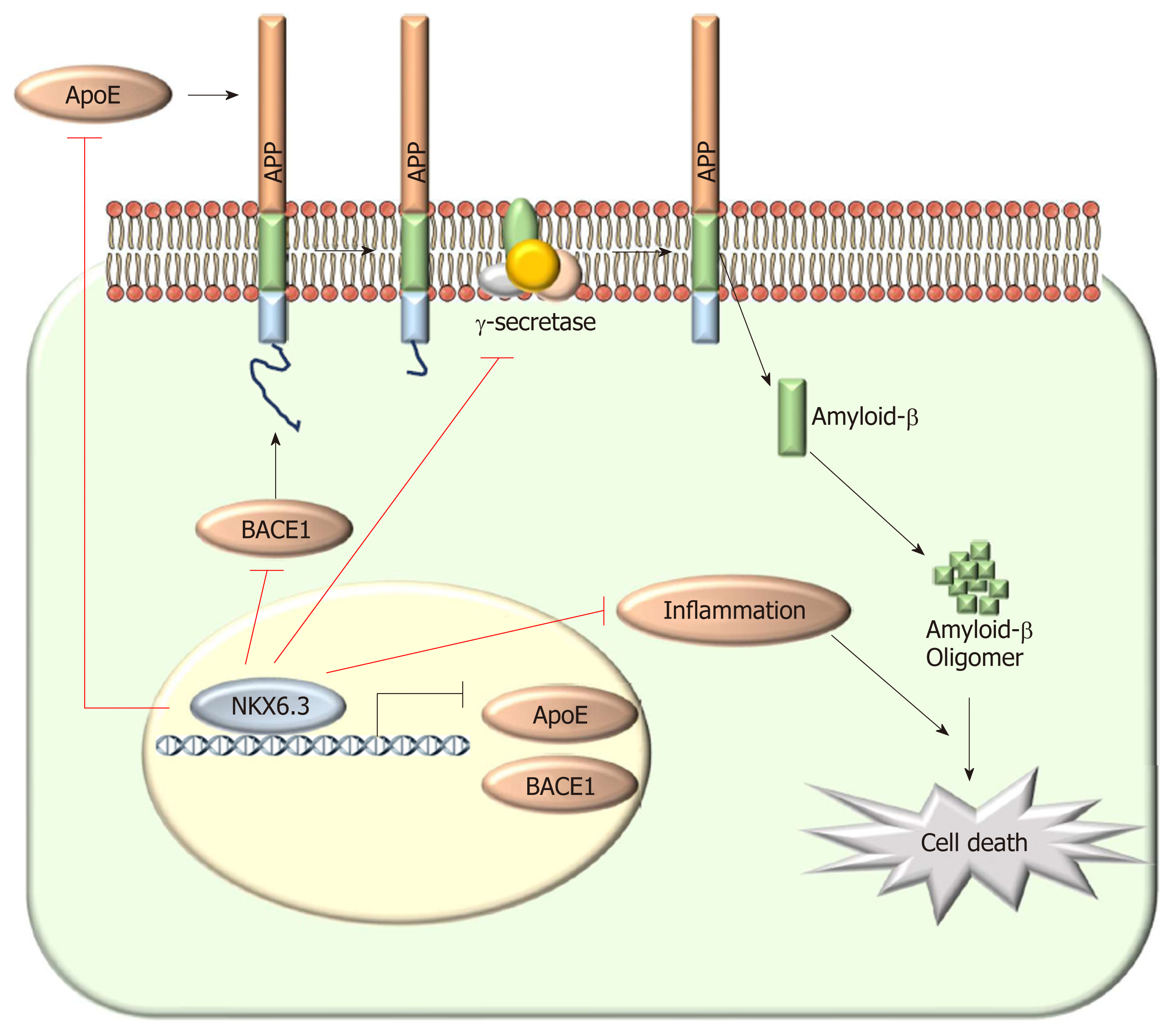
Figure 6 Schematic model of the role of NKX6.
3 in gastric mucosal atrophy. In gastric mucosal cells, NKX6.3 prevents gastric mucosal atrophy by regulating amyloid β (Aβ) peptide accumulation and oligomerization through inhibiting the γ-secretase complex formation and the expression of apolipoprotein E (ApoE), and β-secretase 1. In addition, NKX6.3 suppresses gastric mucosal inflammation by modulating ApoE-induced nuclear factor kappa B activity and expression of cytokines. Aβ: Amyloid β; ApoE: Apolipoprotein E; Bace1: β-secretase 1; NF-κB: Nuclear factor kappa B.
- Citation: Yoon JH, Lee YS, Kim O, Ashktorab H, Smoot DT, Nam SW, Park WS. NKX6.3 protects against gastric mucosal atrophy by downregulating β-amyloid production. World J Gastroenterol 2019; 25(3): 330-345
- URL: https://www.wjgnet.com/1007-9327/full/v25/i3/330.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i3.330