Copyright
©The Author(s) 2019.
World J Gastroenterol. May 21, 2019; 25(19): 2354-2364
Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2354
Published online May 21, 2019. doi: 10.3748/wjg.v25.i19.2354
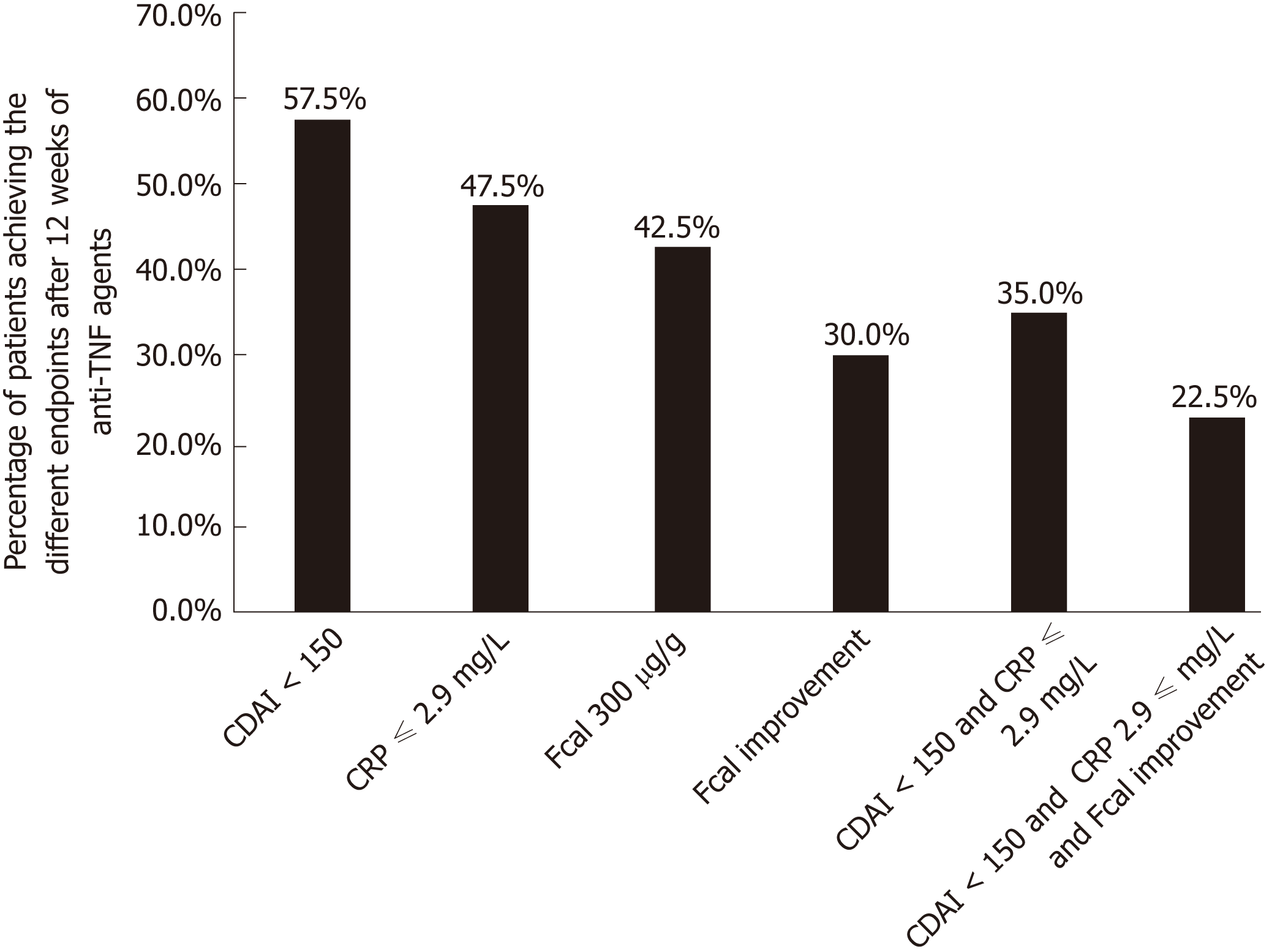
Figure 1 Rate of patients achieving the different therapeutic endpoints after 12 wk of anti-tumor necrosis factor therapy in 40 patients with Crohn’s disease.
CDAI: Crohn’s disease activity index; CRP: C-reactive protein; Fcal: Faecal calprotectin; TNF: Tumor necrosis factor.
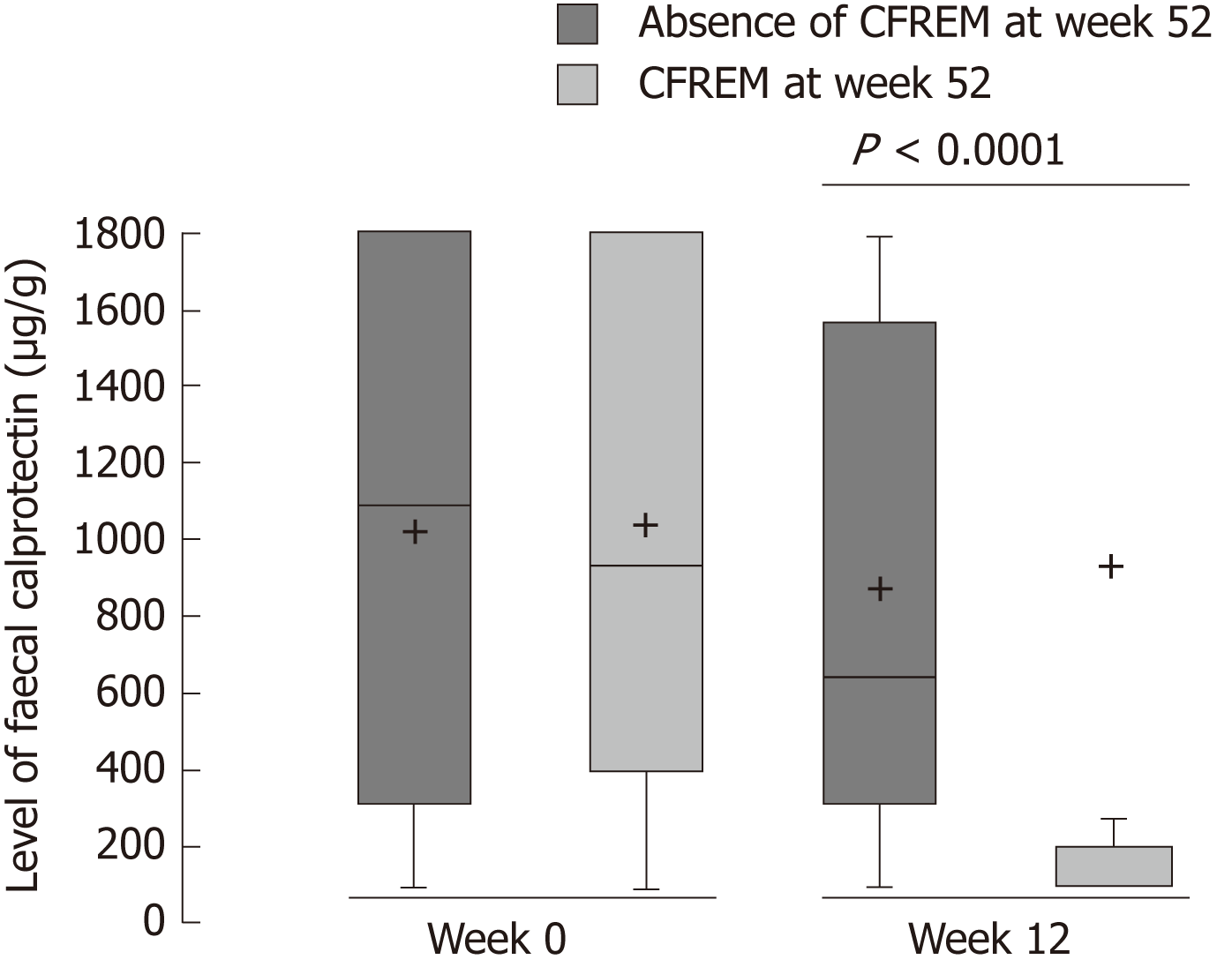
Figure 2 Level of faecal calprotectin at baseline and after 12 wk of anti-tumor necrosis factor therapy in patients with Crohn’s disease achieving steroids-free remission (Crohn’s disease activity index < 150 and C-reactive protein < 2.
9 mg/L and faecal calprotectin < 250 µg/g with no therapeutic intensification and no surgery) or not at week 52. CFREM: Corticosteroids-free remission.
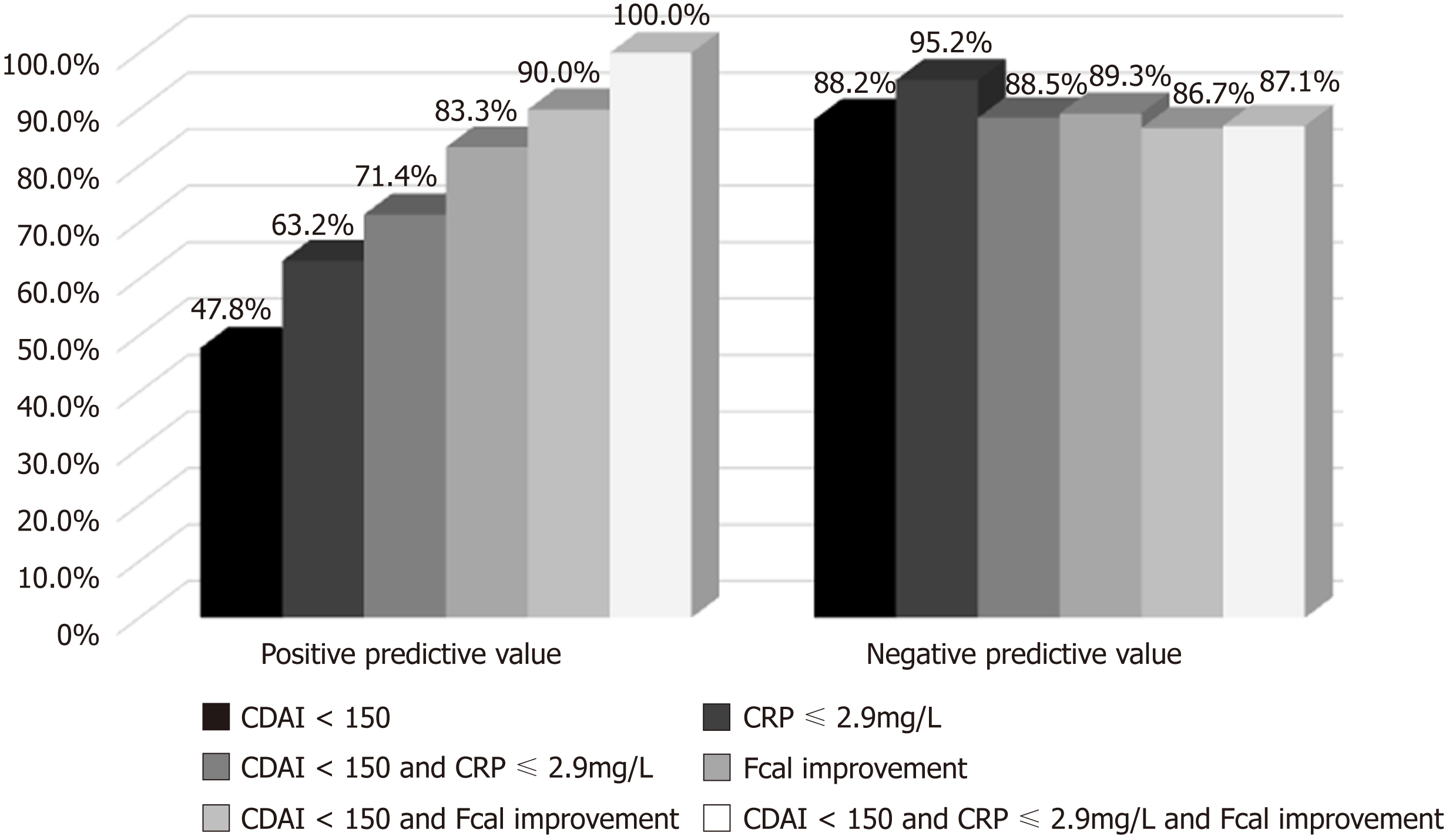
Figure 3 Positive and negative predictive values of Crohn’s disease activity index, C-reactive protein and faecal calprotectin, alone or combined, after 12 wk of anti-tumor necrosis factor to predict corticosteroids-free remission at week 52.
Faecal calprotectin (Fcal) improvement (Fcal < 300 μg/g at W12; or, for patients with initial Fcal < 300 μg/g, at least 50% decrease of Fcal or normalization of Fcal (< 100 μg/g). CDAI: Crohn’s disease activity index; CRP: C-reactive protein; Fcal: Faecal calprotectin.
- Citation: Sollelis E, Quinard RM, Bouguen G, Goutte M, Goutorbe F, Bouvier D, Pereira B, Bommelaer G, Buisson A. Combined evaluation of biomarkers as predictor of maintained remission in Crohn’s disease. World J Gastroenterol 2019; 25(19): 2354-2364
- URL: https://www.wjgnet.com/1007-9327/full/v25/i19/2354.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i19.2354