Copyright
©The Author(s) 2018.
World J Gastroenterol. Dec 7, 2018; 24(45): 5131-5143
Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5131
Published online Dec 7, 2018. doi: 10.3748/wjg.v24.i45.5131
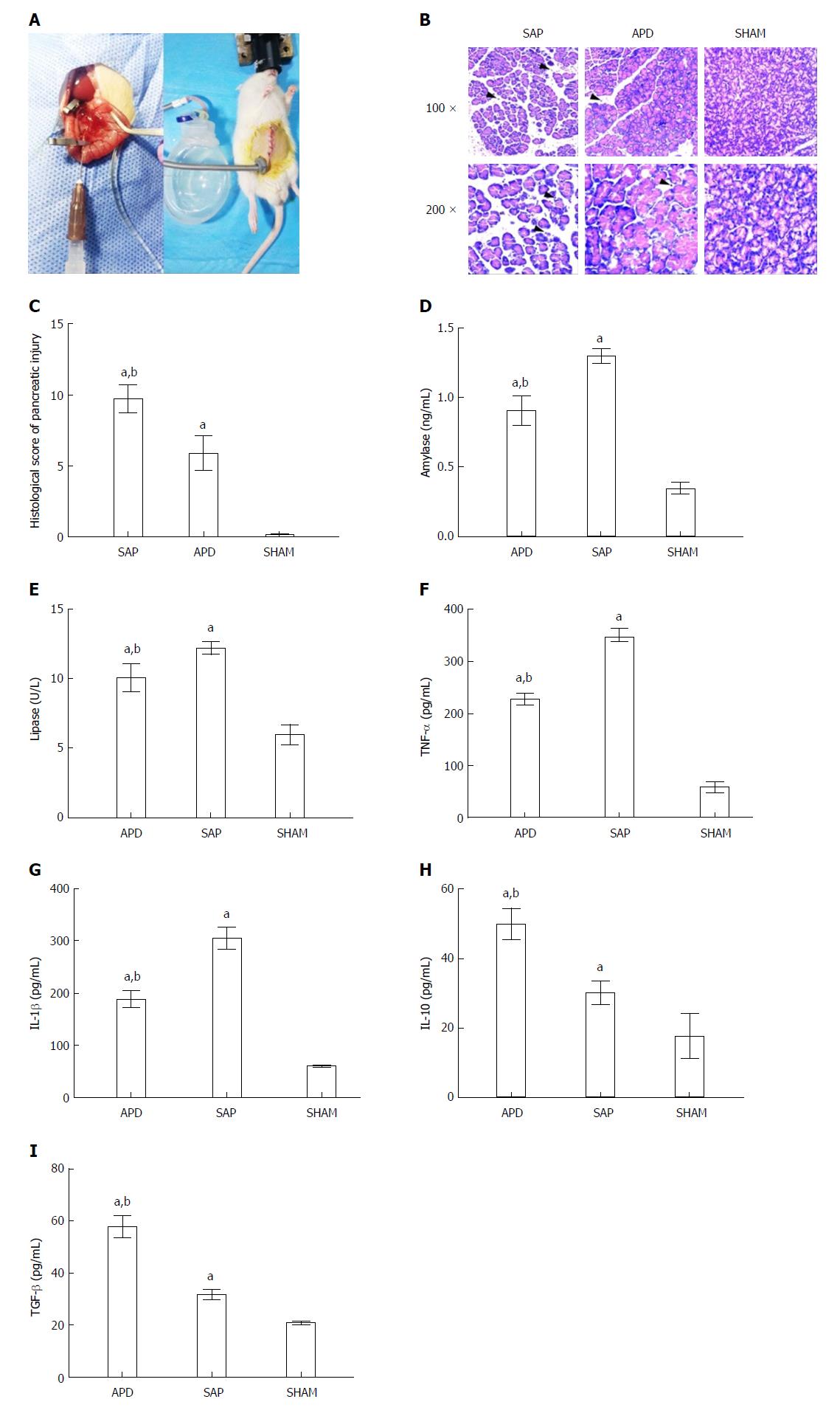
Figure 1 Abdominal paracentesis drainage ameliorates severe acute pancreatitis in a rat model.
A: Model establishment. Retrograde injection of Na-taurocholate (left) and a rat after abdominal paracentesis drainage (APD) treatment (right); B and C: Histopathological analysis of the pancreas. Comprehensive disruption of the pancreatic structure with widespread infiltration of leukocytes, acinar cell vacuolization and necrosis was observed in severe acute pancreatitis (SAP) rats; localized leukocyte infiltration and relatively intact acinar structure were observed in APD rats; D-I: Plasma levels of amylase, lipase, tumor necrosis factor-α, interleukin (IL)-1β, IL-10 and transforming growth factor-β, respectively. Data indicate the mean ± SD of six mice (C-I). aP < 0.05 vs sham, bP < 0.05 vs SAP.
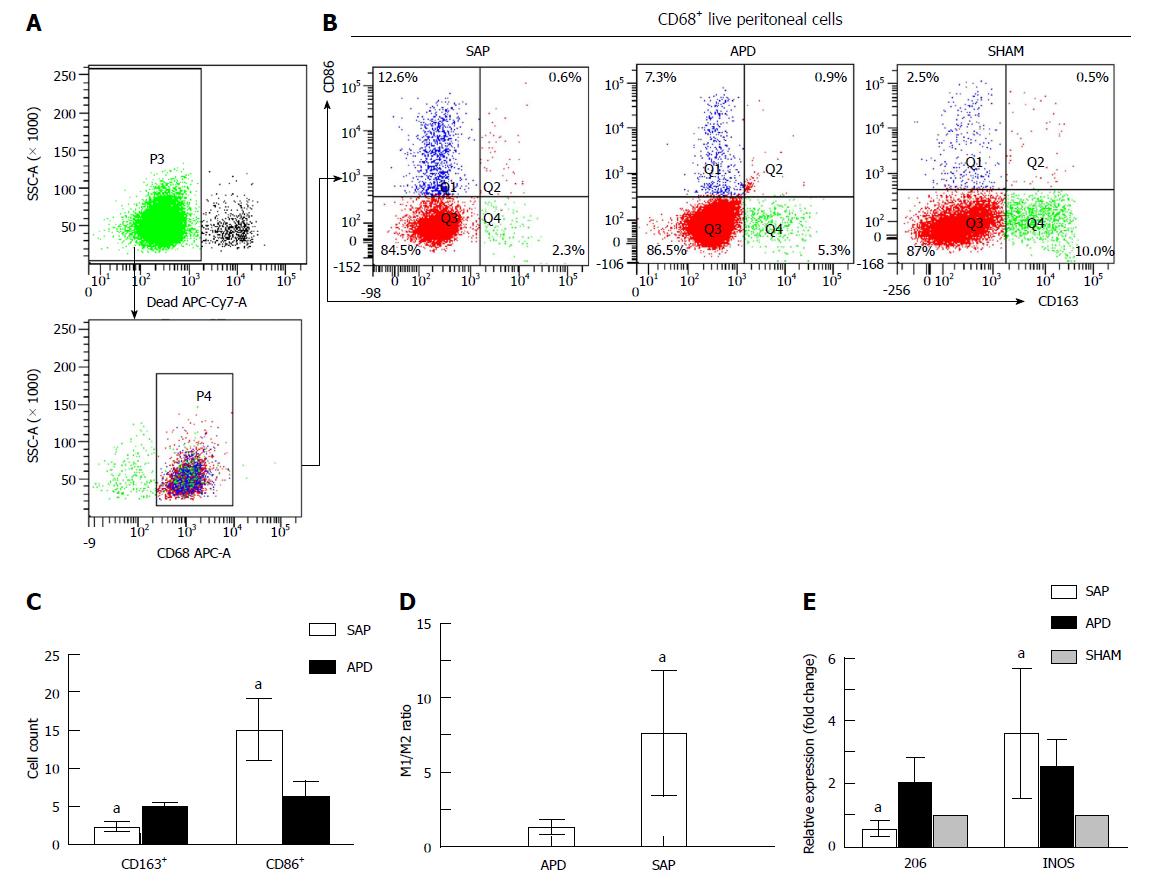
Figure 2 Different polarized phenotypes of peritoneal macrophages in each group.
A: Gating strategy for the peritoneal macrophage population; B-D: Representative dot plot (B) and the percentages (C) and M1/M2 ratio (D) of CD68+CD86+ (M1) cells and CD68+CD163+ (M2) cells in each group; E: Relative expression levels of CD206 and iNOS gene in peritoneal cells measured by real-time PCR and normalized to GAPDH mRNA. The data represent at least three independent experiments (A-B) or indicate the mean ± SD of six mice (E). aP < 0.05 vs abdominal paracentesis drainage.
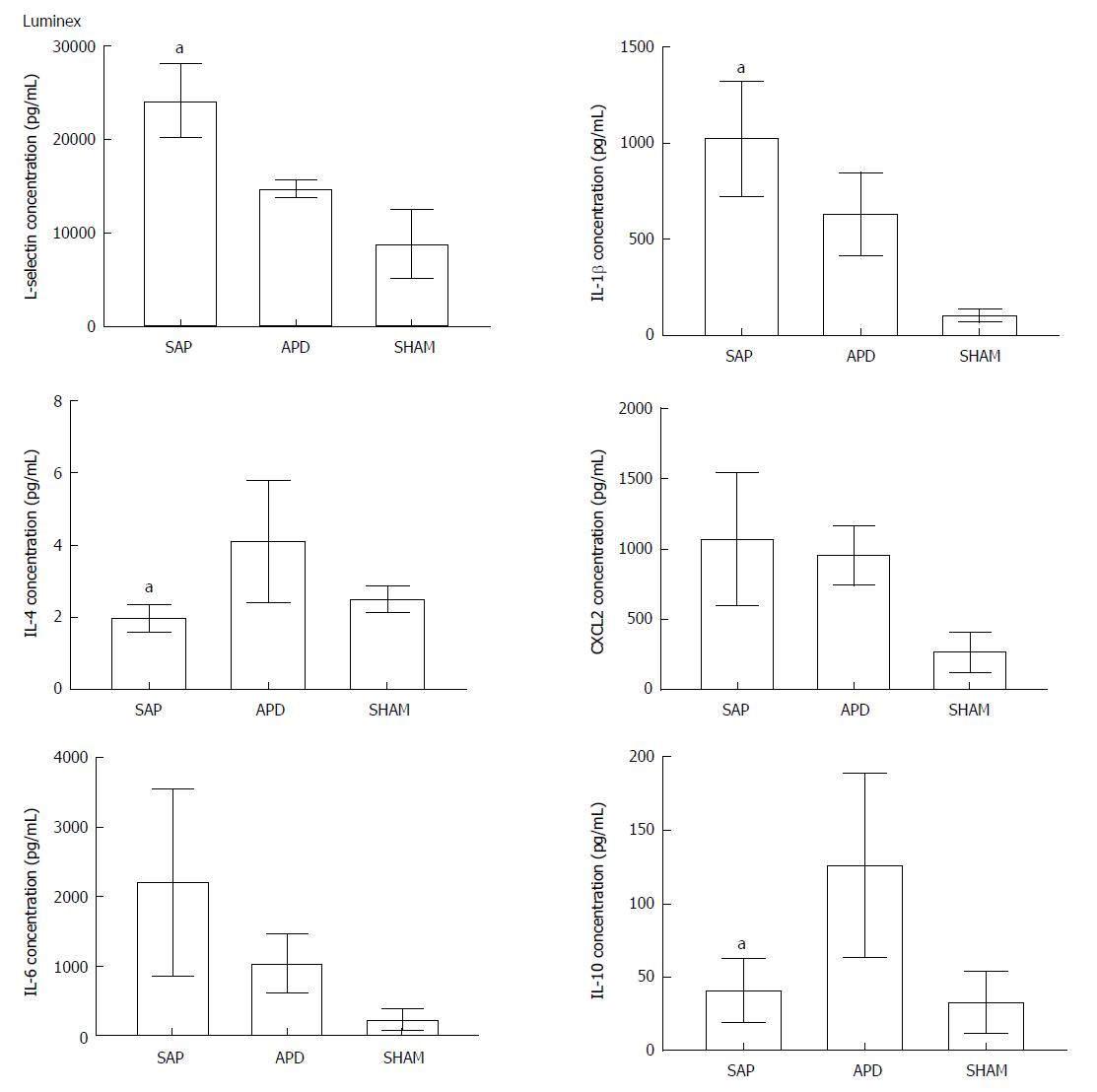
Figure 3 Abdominal paracentesis drainage alters the inflammatory environment in the peritoneal cavity.
Protein levels in peritoneal lavage were measured by Luminex. The results reflect the mean ± SD obtained from six animals in each group. aP < 0.05 vs abdominal paracentesis drainage.
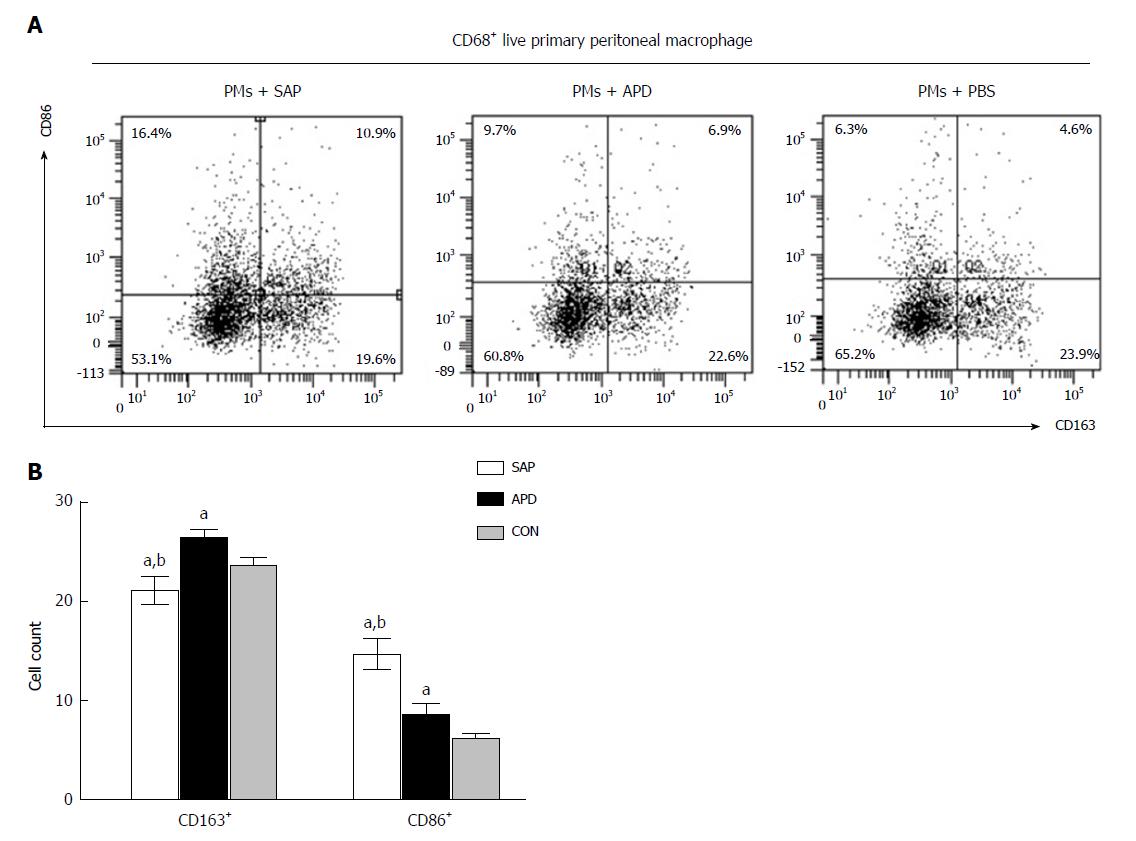
Figure 4 In vitro simulated peritoneal inflammatory environment of abdominal paracentesis drainage rats changes the polarized phenotype of peritoneal macrophages.
Primary peritoneal macrophages were cultured in medium simulating different inflammatory environments. The percentages of CD86+ and CD163+ cells were measured by flow cytometry. The data are representative of at least three independent experiments. aP < 0.05 vs CON, bP < 0.05 vs abdominal paracentesis drainage.
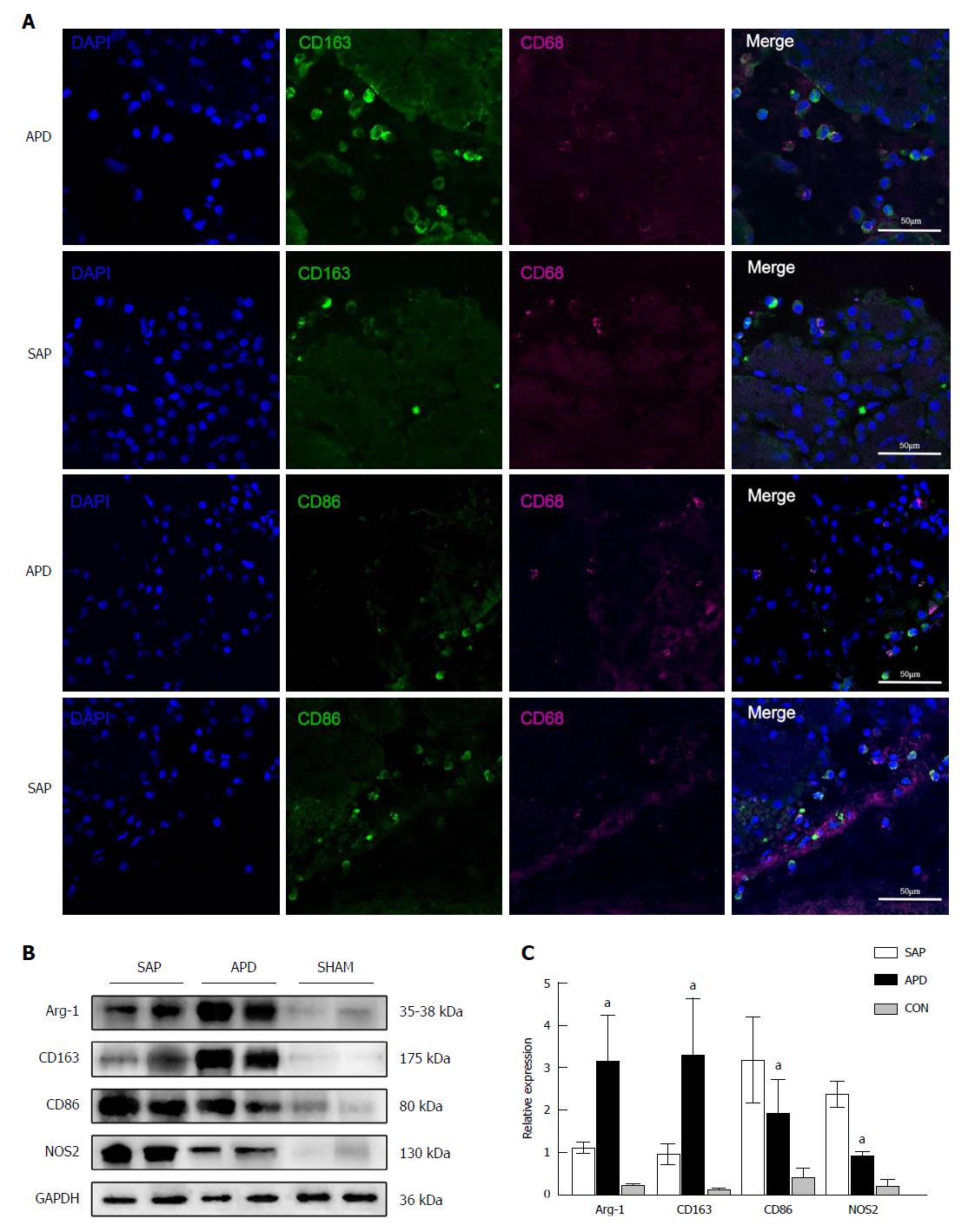
Figure 5 Number of M2 macrophages increases in the pancreas of abdominal paracentesis drainage rats and exerts anti-inflammatory effects.
A: Pancreatic tissues from each group were stained with DAPI (blue), CD163 (green in upper two panels), CD86 (green in lower two panels) and CD68 (infrared represented by carmine). Representative images are shown; B and C: Protein levels of Arg-1, CD163, CD86 and iNOS in the pancreatic tissues of each group were measured by Western blot, and the relative expression of these proteins was normalized to GAPDH. Data are representative of at least three independent experiments. aP < 0.05 vs severe acute pancreatitis.
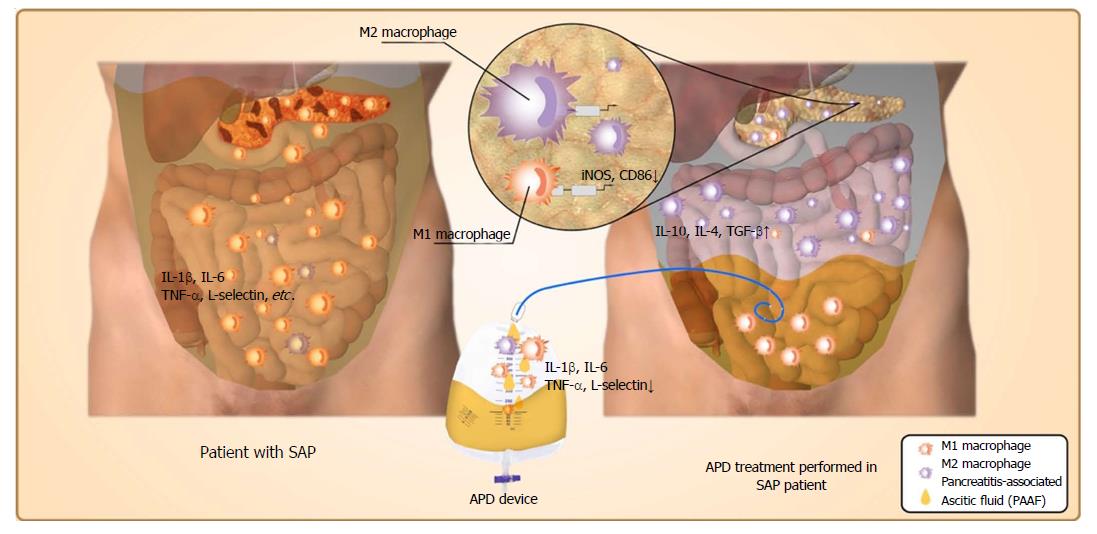
Figure 6 Possible mechanisms responsible for the beneficial effects of abdominal paracentesis drainage on severe acute pancreatitis.
Once severe acute pancreatitis (SAP) occurs, the inflammatory cells are activated following acinar cell injuries and exudate full of pro-inflammatory mediators collects in the peritoneal cavity, which can polarize the peritoneal macrophages (PMs) towards the M1 phenotype and lead to the overexpression of pro-inflammatory mediators by PMs. By removing the pancreatitis-associated ascitic fluids, abdominal paracentesis drainage (APD) could improve the inflammatory environment of the peritoneal cavity, thus promoting M2 polarization of PMs in the peritoneal cavity. Meanwhile, APD could also promote M2 polarization of macrophages in pancreatic tissues. These events could upregulate the expression of anti-inflammatory cytokines, which ultimately ameliorate pancreatic injury. APD: Abdominal paracentesis drainage; SAP: Severe acute pancreatitis.
- Citation: Liu RH, Wen Y, Sun HY, Liu CY, Zhang YF, Yang Y, Huang QL, Tang JJ, Huang CC, Tang LJ. Abdominal paracentesis drainage ameliorates severe acute pancreatitis in rats by regulating the polarization of peritoneal macrophages. World J Gastroenterol 2018; 24(45): 5131-5143
- URL: https://www.wjgnet.com/1007-9327/full/v24/i45/5131.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i45.5131