Copyright
©The Author(s) 2018.
World J Gastroenterol. Jan 21, 2018; 24(3): 351-359
Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.351
Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.351
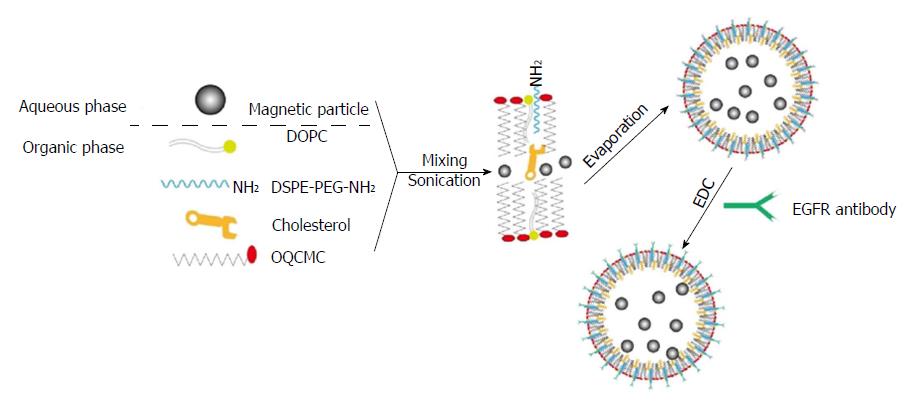
Figure 1 Schematic diagram showing the preparation of epidermal growth factor receptor-targeted immune magnetic liposomes with hydrophilic and hydrophobic magnetic nanoparticles.
EGFR: Epidermal growth factor receptor.
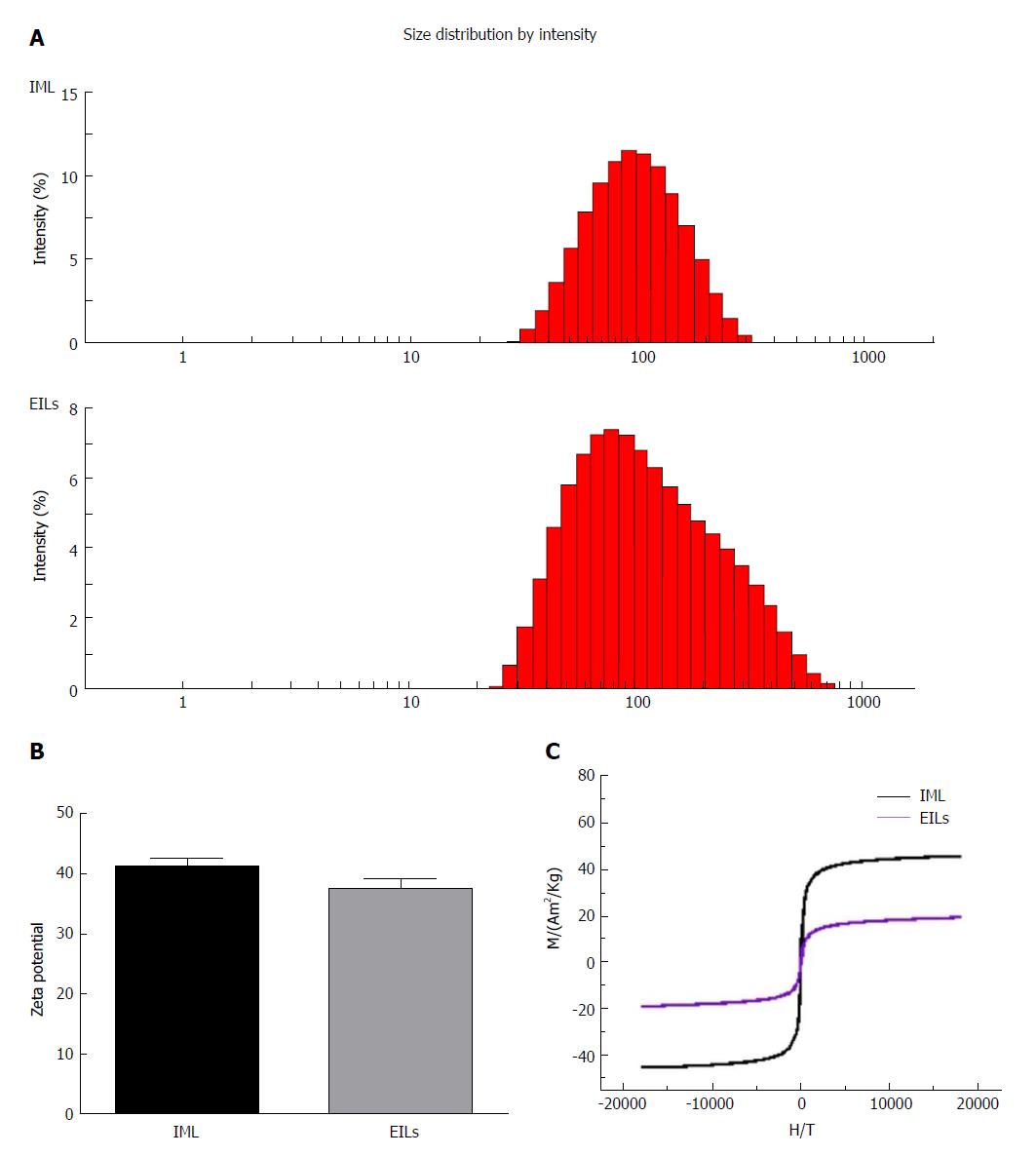
Figure 2 Characterization of epidermal growth factor receptor-targeted immune magnetic liposomes.
The hydrodynamic diameter (A), zeta potential (B), and magnetization curves (C) at 300 K of magnetic polymeric liposomes are shown. IML: Immune magnetic liposomes; EGFR: Epidermal growth factor receptor; EILs: EGFR-targeted immune magnetic liposomes.
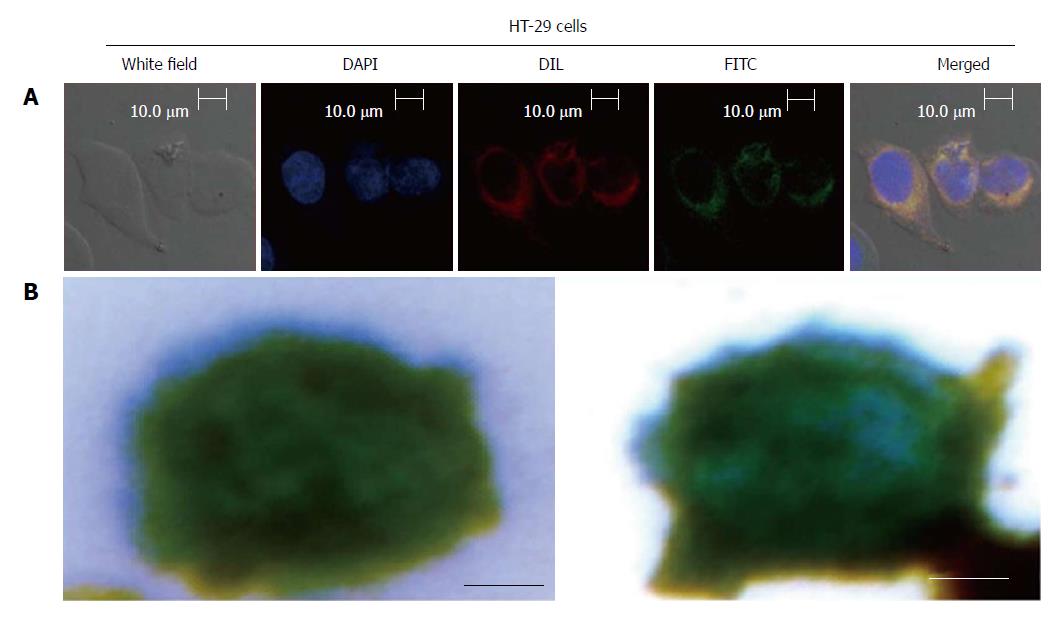
Figure 3 Size of the captured circulating tumor cells and color of stained magnetic beads.
A: Localization and distribution of EILs in HT-29 cells. Cells were cultured in FITC-labeled EILs-containing medium on bottom of a glass dish (35 mm dish with 14 mm bottom wells) for 30 min followed by treatment with DAPI dye and Dil dye for 5-10 min, separately, and subsequent examination by confocal microscopy; B: Prussian blue staining of EGFR IMLs with HT-29 Cells. (Scale bar: 5 μm). IMLs: Immune magnetic liposomes; EILs: EGFR-targeted immune magnetic liposomes.
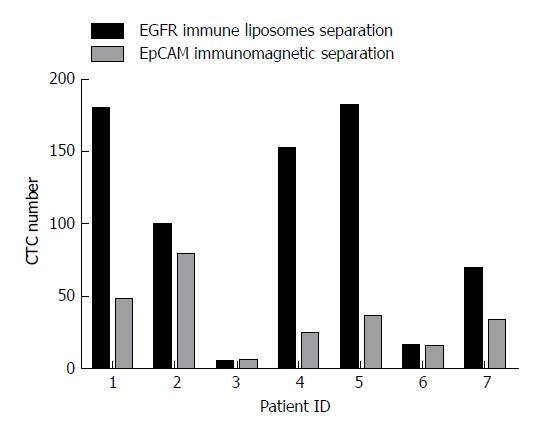
Figure 4 The number of circulating tumor cells captured by epithelial cell adhesion molecule magnetic beads and epidermal growth factor receptor-targeted immune magnetic liposomes.
Side-by-side representation of circulating tumor cell (CTC) enumeration results obtained from our integrated CTC-capture method (normalized to 7.5 mL of blood) with EGFR immune magnetic liposomes and epithelial cell adhesion molecule (EpCAM) immunomagnetic beads on matched samples from seven patients.
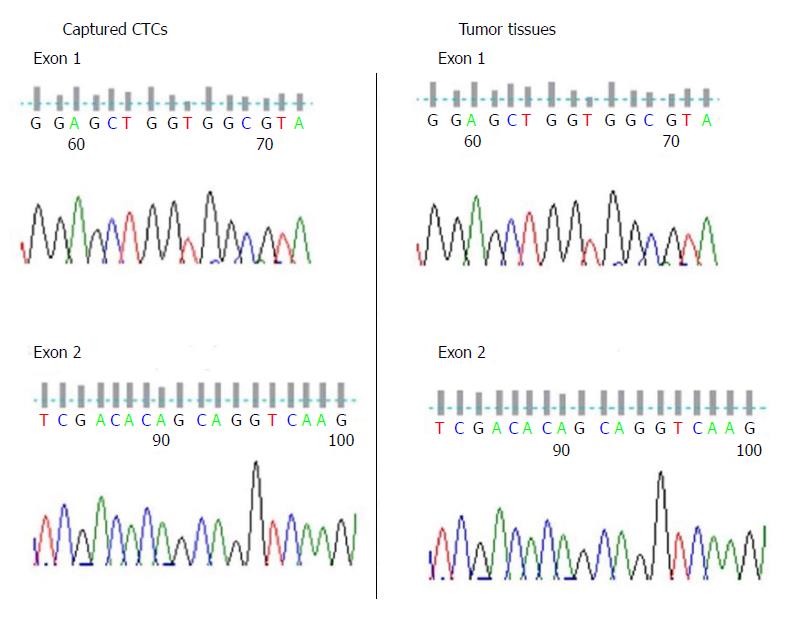
Figure 5 Gene mutation test of KRAS for captured circulating tumor cells and tumor tissue.
CTCs: Circulating tumor cells.
- Citation: Kuai JH, Wang Q, Zhang AJ, Zhang JY, Chen ZF, Wu KK, Hu XZ. Epidermal growth factor receptor-targeted immune magnetic liposomes capture circulating colorectal tumor cells efficiently. World J Gastroenterol 2018; 24(3): 351-359
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.351